Abstract
Platelets play a central role in primary hemostasis by forming aggregates that plug holes in injured vessels. Half a century ago, detailed studies of the microvasculature by electron microscopy revealed that under inflammatory conditions that do not induce major disruption to vascular structure, individual platelets are mobilized to the vessel wall, where they interact with leukocytes and appear to seal gaps that arise between endothelial cells. Recent developments in genetic engineering and intravital microscopy have allowed further molecular and temporal characterization of these events. Surprisingly, it turns out that platelets support the recruitment of leukocytes to sites of inflammation. In parallel, however, they exercise their hemostatic function by securing the integrity of inflamed blood vessels to prevent bleeding from sites of leukocyte infiltration. It thus appears that platelets not only serve in concert as building blocks of the hemostatic plug but also act individually as gatekeepers of the vascular wall to help preserve vascular integrity while coordinating host defense. Variants of this recently appreciated hemostatic function of platelets that we refer to as “inflammation-associated hemostasis” are engaged in different contexts in which the endothelium is challenged or dysfunctional. Although the distinguishing characteristics of these variants and the underlying mechanisms of inflammation-associated hemostasis remain to be fully elucidated, they can differ notably from those supporting thrombosis, thus presenting therapeutic opportunities.
Platelets and endothelial cells interact to safeguard vascular integrity
The term “vascular integrity” is variably used to refer to the capacity of the endothelium to retain fluids, macromolecules, and leukocytes1,2 ; to that of the vessel as a whole to structurally retain all components of blood3 ; or more generally to the capacity of the vessel to properly exert all its normal functions including the regulation of blood flow.4 Although the endothelium controls active and passive passage of fluids, cells, and macromolecules, platelets are recruited if defects in the endothelium or structural components of the vessel wall, including the endothelial basement membrane, the internal elastic lamina, and mural cells, endanger these functions and the retention of red blood cells. As excessive responses may instead jeopardize vascular patency, extensive crosstalk among the endothelium, platelets, and coagulation and fibrinolytic systems ensure that protective responses are fine-tuned to the challenge at hand. In this regard, it is noteworthy that platelets, usually considered as components of hemostatic plugs and occlusive thrombi, are also recruited to protect the vessel wall in an individual manner, without posing inappropriate risk for thrombosis when the vessel is intact but the endothelial barrier is rendered dysfunctional in conditions such as cancer, inflammation, and radiation injury. In this review, we discuss how historical electron microscopy (EM) and recent intravital microscopy (IVM) studies have shed light on this lesser known function of platelets.
Inflammation recruits platelets to leukocytes and the vessel wall
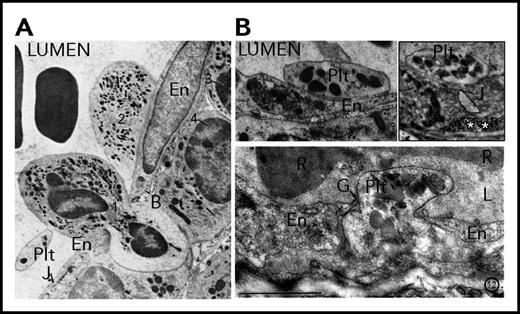
Descriptions and images of the interactions between platelets and inflamed vessels can be found in abundance in studies dating back to the golden age of cellular EM. For example, in their 1961 study on the effect of histamine and serotonin on vascular permeability, when describing the localization of platelets at sites of vascular leaks and endothelial gaps, Majno and Palade wrote that “platelets were found frequently and at all levels: sometimes bridging a gap, sometimes plugging it or lying deeper inside the vascular wall.”5 Their observations were illustrated by images showing single platelets lodged in gaps within the endothelial monolayer and covering the basement membrane (Figure 1). A few years later, Cotran and Majno made similar observations in a model of thermal injury. In addition to single platelets filling gaps, they further described the presence of platelets adherent to the endothelium contiguous with gaps, or converging on both sides of these openings.6 Contemporary studies focusing on neutrophil emigration reported the binding of platelets to, and colocalization of platelets adherent to the surface of the endothelium with, emigrating leukocytes7-9 (Figure 1). These observations showing that “intercellular endothelial gaps were sometimes bridged or surrounded by platelets” led Cotran to propose more than 50 years ago that “at least one of the endothelial-supporting functions of the platelets is to plug microscopic holes after they are formed.”6 Intriguingly, most of the platelets shown interacting with the activated endothelium, neutrophils, or basement membrane in these studies display residual granules, indicating a limited state of activation. Marchesi8,9 and others5,7 also described the presence of platelets and platelet fragments in the cytoplasm of endothelial cells at sites of neutrophil extravasation, suggesting the ability of endothelial cells to phagocytose platelets and platelet microparticles, a phenomenon since confirmed for endothelial cells as well as neutrophils both in vitro and in vivo.10-14
Platelet interactions with the vessel wall at sites of increased permeability and neutrophil transmigration. (A) A platelet bound to an emigrating neutrophil squeezing between 2 adjacent endothelial cells in an inflamed rat mesenteric venule. Adapted from Marchesi and Florey9 with permission. (B, upper left) A platelet can be seen adherent to the surface of the inflamed endothelium. Adapted from Marchesi8 with permission. (Upper right) A single platelet that seems to be sealing an open endothelial junction in an inflamed venule of the rat cremaster muscle. The white asterisks indicate the subendothelial accumulation of intravenously injected colloidal mercuric sulfide particles used as a marker of endothelial permeability. (Bottom) A single platelet filling an endothelial gap. Note that this platelet has lost its discoid shape, molding the interendothelial space. Adapted from Majno and Palade65 with permission. B, basement membrane; En, endothelial cell; G, endothelial gap; J, junction; L, lumen; Plt, platelet; R, red blood cell.
Platelet interactions with the vessel wall at sites of increased permeability and neutrophil transmigration. (A) A platelet bound to an emigrating neutrophil squeezing between 2 adjacent endothelial cells in an inflamed rat mesenteric venule. Adapted from Marchesi and Florey9 with permission. (B, upper left) A platelet can be seen adherent to the surface of the inflamed endothelium. Adapted from Marchesi8 with permission. (Upper right) A single platelet that seems to be sealing an open endothelial junction in an inflamed venule of the rat cremaster muscle. The white asterisks indicate the subendothelial accumulation of intravenously injected colloidal mercuric sulfide particles used as a marker of endothelial permeability. (Bottom) A single platelet filling an endothelial gap. Note that this platelet has lost its discoid shape, molding the interendothelial space. Adapted from Majno and Palade65 with permission. B, basement membrane; En, endothelial cell; G, endothelial gap; J, junction; L, lumen; Plt, platelet; R, red blood cell.
These historical studies are pertinent to recent studies indicating unique roles for platelets in inflammation-associated hemostasis that differ from classical hemostasis in mechanisms of engagement.
Platelets continuously prevent bleeding during inflammation and infection
Almost a decade ago, Denisa Wagner’s group identified inflammatory reactions as a possible cause of bleeding during thrombocytopenia. When mice were rendered severely thrombocytopenic with a platelet-depleting antibody and subsequently challenged in models of dermatitis, acute lung inflammation, or cerebral ischemia/reperfusion (IR),15 bleeding was observed and limited to inflamed organs. Sensitization was also reported in models of viral16-18 and bacterial19 infection, radiation injury,20,21 and solid tumors.22-24 Thus, platelets are needed to prevent bleeding during inflammation/infection, mobilization of adaptive immunity, reperfusion injury, and radiation, as well as in the tumor vasculature (Table 1).
Prevention of inflammatory bleeding by platelets
| . | Bleeding phenotype . | Bleeding cause . | Primary pathways for bleeding prevention . | Dispensable/secondary pathways for bleeding prevention . | References . | |
|---|---|---|---|---|---|---|
| 100% platelets . | <2.5% platelets . | |||||
| Abnormal inflammatory bleeding | ||||||
| Immune complex-induced dermatitis (rpA) | No bleeding | Bleeding | Neutrophils | GPVI, (CLEC-2) | GPIb, VWF, GPIIIbIIIa, (P2Y12), (TXA2), (PAR4), S1P, platelet granules | 15, 20, 21, 32, 33, 34, 67, 92 |
| Irritant contact dermatitis | No bleeding | Bleeding | Neutrophils | ND | ND | 15, 20 |
| UVB-induced dermatitis | No bleeding | Bleeding | Neutrophils | ND | ND | 20 |
| LPS-induced lung inflammation | No bleeding | Bleeding | Neutrophils | (GPVI), (CLEC-2) | GPIIbIIIa, S1P, (P2Y12), (TXA2), (PAR4), platelet granules | 15, 21, 32, 33, 63, 92 |
| Klebsiella-induced pneumonia | No bleeding | Bleeding | ND | ND | ND | 19 |
| Immunization (OVA/CFA) | No bleeding | Bleeding | Lymphocytes | CLEC-2, podoplanin | GPIIbIIIa, platelet S1P | 21, 44 |
| Immune complex-induced glomerulonephritis | No bleeding | Bleeding | Neutrophils | ND | GPIb | 25 |
| Cerebral IRI (tMCAO) | No bleeding | Bleeding | ND | GPIIbIIIa, platelet granules | GPVI, GPIb, VWF | 15, 33, 48, 54, 55 |
| Viral infection (LCMV) | No bleeding | Bleeding | ND | GPIIbIIIa | GPIb, P-Selectin | 16, 17, 18 |
| Solid tumors (4T1, B16F10, LLC) | No bleeding | Bleeding | Macrophages and neutrophils | (Platelet granules) | GPIb, VWF, GPIIIbIIIa | 22, 23, 43 |
| No abnormal inflammatory bleeding | ||||||
| Arthritis | No bleeding | No bleeding | NA | NA | NA | 36, 37 |
| Thioglycolate-induced peritonitis | No bleeding | No bleeding | NA | NA | NA | 2 |
| Immune complex-induced peritonitis | No bleeding | No bleeding | NA | NA | NA | 34 |
| Endotoxemia | No bleeding | No bleeding | NA | NA | NA | 35 |
| . | Bleeding phenotype . | Bleeding cause . | Primary pathways for bleeding prevention . | Dispensable/secondary pathways for bleeding prevention . | References . | |
|---|---|---|---|---|---|---|
| 100% platelets . | <2.5% platelets . | |||||
| Abnormal inflammatory bleeding | ||||||
| Immune complex-induced dermatitis (rpA) | No bleeding | Bleeding | Neutrophils | GPVI, (CLEC-2) | GPIb, VWF, GPIIIbIIIa, (P2Y12), (TXA2), (PAR4), S1P, platelet granules | 15, 20, 21, 32, 33, 34, 67, 92 |
| Irritant contact dermatitis | No bleeding | Bleeding | Neutrophils | ND | ND | 15, 20 |
| UVB-induced dermatitis | No bleeding | Bleeding | Neutrophils | ND | ND | 20 |
| LPS-induced lung inflammation | No bleeding | Bleeding | Neutrophils | (GPVI), (CLEC-2) | GPIIbIIIa, S1P, (P2Y12), (TXA2), (PAR4), platelet granules | 15, 21, 32, 33, 63, 92 |
| Klebsiella-induced pneumonia | No bleeding | Bleeding | ND | ND | ND | 19 |
| Immunization (OVA/CFA) | No bleeding | Bleeding | Lymphocytes | CLEC-2, podoplanin | GPIIbIIIa, platelet S1P | 21, 44 |
| Immune complex-induced glomerulonephritis | No bleeding | Bleeding | Neutrophils | ND | GPIb | 25 |
| Cerebral IRI (tMCAO) | No bleeding | Bleeding | ND | GPIIbIIIa, platelet granules | GPVI, GPIb, VWF | 15, 33, 48, 54, 55 |
| Viral infection (LCMV) | No bleeding | Bleeding | ND | GPIIbIIIa | GPIb, P-Selectin | 16, 17, 18 |
| Solid tumors (4T1, B16F10, LLC) | No bleeding | Bleeding | Macrophages and neutrophils | (Platelet granules) | GPIb, VWF, GPIIIbIIIa | 22, 23, 43 |
| No abnormal inflammatory bleeding | ||||||
| Arthritis | No bleeding | No bleeding | NA | NA | NA | 36, 37 |
| Thioglycolate-induced peritonitis | No bleeding | No bleeding | NA | NA | NA | 2 |
| Immune complex-induced peritonitis | No bleeding | No bleeding | NA | NA | NA | 34 |
| Endotoxemia | No bleeding | No bleeding | NA | NA | NA | 35 |
Overview of the experimental models of inflammation in which immunodepletion of platelets (<2.5% platelets) was reported to cause abnormal inflammatory bleeding or not. For each model, when known, the causes of inflammatory bleeding as well as the pathways involved or not for the prevention of bleeding are indicated. Candidate pathways whose role was investigated using platelet adoptive transfer methods based on platelet transfusion experiments are indicated in parentheses and italics.
IRI, ischemia-reperfusion injury; LCMV, lymphocytic choriomeningitis virus, not determined; LPS, lipopolysaccharide; NA, not applicable; OVA/CFA, ovalbumin/complete Freund's adjuvant; rpA, reverse passive Arthus reaction; tMCAO, transient middle cerebral artery occlusion.
IVM observation of the skin microcirculation during the immunoglobulin G (IgG)–mediated reverse passive Arthus reaction (rpA) revealed that inflammatory bleeding in thrombocytopenic mice started as early as 20 minutes after induction.15 The early requirement for platelets in controlling bleeding during inflammation was also described in a model of glomerulonephritis.25 In this model, platelet accumulation occurred long before evidence of thrombosis, and platelet depletion before induction exacerbated glomerular damage and hemorrhage. Thus, platelets intervene to prevent bleeding from the very onset of the inflammatory response.
These observations made in experimental models may suggest that so-called “spontaneous” bleeding events observed in thrombocytopenic patients may not be a consequence uniquely of low platelet counts but also involve immune sensitization of the vessel. In support of this notion, a case report described petechial bleeding accompanying acute skin inflammation secondary to a first-degree sunburn in a patient with mild thrombocytopenia.26 Similar to in thrombocytopenic mice, bleeding in this patient was restricted to the inflamed skin. This incidental observation was reproduced experimentally in a study in which the exposure of the skin of thrombocytopenic patients to controlled doses of ultraviolet B light was shown to trigger petechial bleeding restricted to the irradiated area in response to ultraviolet B doses at which no bleeding was observed in control subjects.20 Other reports support a relation between inflammation and bleeding in thrombocytopenia: glucocorticoids prevented spontaneous and IR-induced petechial formation in thrombocytopenic patients irrespective of improvement in platelet counts,27,28 leukopenia was associated with a decreased risk of bleeding in thrombocytopenic oncology patients,29 and low platelet count as well as high levels of the inflammation marker C-reactive protein predicted symptomatic hemorrhagic transformation in nonlacunar acute ischemic stroke.30 More direct evidence comes from the increased risk for severe bleeding in patients with thrombocytopenia and/or platelet dysfunction consecutive to viral infections (eg, viral hemorrhagic fevers, Epstein-Barr virus, or acquired immunodeficiency syndrome).31
Inflammation-induced bleeding in the skin, brain, lungs, and kidneys of thrombocytopenic mice has been confirmed by different research groups15,19-21,25,32-34 and could suggest that platelets are needed to prevent bleeding in all inflamed organs. Yet no bleeding was observed in thrombocytopenic mice subjected to the rpA (which caused skin bleeding when elicited subcutaneously or intradermally15,32-34 ) when induced in the peritoneal cavity.34 Furthermore, there was no report of bleeding in studies focusing on endothelial permeability and in which platelet-depleted or platelet-deficient mice were challenged with thioglycolate-induced peritonitis,2 endotoxemia,35 or autoimmune rheumatoid arthritis36,37 (Table 1). It thus appears that the need for platelets to prevent bleeding varies with the organ affected and/or with the cause or level of inflammation.
Inflammation-associated hemostasis is sustained by nonaggregated platelets
Inflammation-associated hemostasis displays some characteristic features. First, bleeding is only observed with profound thrombocytopenia, as 15%, 10%, or 2% of normal mouse platelet counts, respectively, were sufficient to prevent bleeding during lymphocytic choriomeningitis virus infection,16,17 the cutaneous rpA,15 and lung infection with Klebsiella pneumonia.19 This suggests a mechanism requiring a limited quantity of platelets and may be of clinical relevance, as it suggests it may not take many platelets to rescue inflammation-induced bleeding in patients with thrombocytopenia. It should be noted that humans have fewer platelets than mice (100 000-400 000 vs 600 000-1 000 000/µL blood) and may be sensitized at lower relative deficiency if the absolute platelet concentration is the key threshold determinant. Other inflammatory functions of platelets are more sensitive to reductions in platelet counts than inflammation-associated hemostasis, as moderate thrombocytopenia can decrease inflammation-induced endothelial permeability and/or leukocyte extravasation without causing hemorrhage.38-40 Different threshold requirements for platelet concentrations for different functions of platelets during inflammation hint at mechanistic distinctions that could potentially be targeted independently for therapeutic benefit.
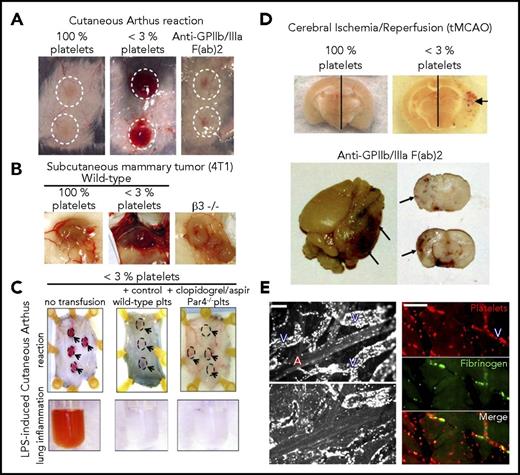
The low platelet concentration threshold may be explained by another characteristic feature of inflammation-associated hemostasis: that it does not engage the classical hemostatic pathways if inflammation or injury is successfully contained (Table 1). Platelet aggregation through GPIIb/IIIa is essential for hemostasis, as illustrated by Glanzmann’s thrombasthenia, a rare bleeding disorder caused by absence or highly reduced expression of this integrin.41,42 However, neither antibody-induced nor genetically induced deficiency in GPIIb/IIIa or in CalDAG-GEFI, a signaling molecule essential for its activation, resulted in bleeding during the cutaneous rpA (Figure 2A).15,32,33 Absence of GPIIb/IIIa also did not cause bleeding in solid tumors (Figure 2B), whose inflammatory stroma make them prone to bleeding in thrombocytopenia,22,23,43 nor did its inhibition induce bleeding in draining lymph nodes after immunization, when platelets are needed to prevent bleeding at high endothelial venules.44 Although primary adhesion of platelets to the injured vessel wall is largely independent of G protein-coupled receptor (GPCR) signaling, the subsequent recruitment of additional platelets and their aggregation require GPCR agonists such as adenosine 5′-diphosphate, thromboxane A2, or thrombin,45,46 which trigger signaling pathways that enhance platelet granule secretion and cross-linking through activation of GPIIb/IIIa. The role of platelet GPCRs in inflammation-associated hemostasis was addressed in a study that used adoptive platelet transfer to generate chimeric animals with platelet-specific defects.47 In this model, severe thrombocytopenia was induced in transgenic mice expressing a chimeric hIL4Rα/GPIbα protein instead of GPIbα on the platelet surface, using antibodies against human IL4Rα. Platelet-depleted hIL4Rα/GPIbα transgenic mice could then be transfused with any type of donor platelets, as the cytotoxicity of the platelet-depleting antibody to human IL4Rα was limited to hIL4Rα/GPIbα platelets. Using this strategy, it was shown that although unresponsive to ADP, thrombin, and thromboxane A2, aspirin and clopidogrel-treated PAR4−/− platelets retained their capacity to rescue bleeding in inflamed skin and lungs32 (Figure 2C; Table 1).
Prevention of inflammatory bleeding by platelets can occur independent of G protein–coupled receptors and GPIIbIIIa. (A) Representative images of cutaneous rpa spots showing that, unlike thrombocytopenic mice (<3% platelets), mice treated with a blocking F(ab')2 fragment antibody to GPIIb/IIIa did not develop bleeding in the inflamed skin. Adapted from Deppermann et al.33 (B) Representative images of mouse mammary tumor showing that, unlike thrombocytopenic mice (<3% platelets), mice with a genetic deficiency in β 3 integrins (β3−/−) did not develop tumor bleeding. Adapted from Ho-Tin-Noé et al49 with permission. (C) Transgenic hIL-4Rα/GPIbα mice were immunodepleted for platelets and transfused or not with control wild-type platelets or with platelets isolated from clopidogrel and aspirin-treated PAR4−/− mice. Those mice were then challenged in the skin with the rpA or in the lungs with LPS, as indicated. Representative images of the rpa site (dashed outlines and arrows) and of the bronchoalveolar lavage fluid are shown. Remarkably, just like control wild-type platelets, clopidogrel- and aspirin-treated PAR4−/− platelets protected against inflammatory bleeding in the inflamed skin and lungs. Adapted from Boulaftali et al32 with permission. (D) Representative images showing brain sections from mice that were subjected to tMCAO. The photographs were taken 24 hours after reperfusion. Hemorrhagic transformation under the form of petechial bleeding developed in the ischemic hemisphere of thrombocytopenic mice (<3% platelets) and of mice treated with a blocking F(ab')2 fragment antibody to GPIIb/IIIa. (Upper) Adapted from Goerge et al.32 (Lower) Adapted from Kleinschnitz et al.48 (E) Intravital imaging of cortical microvessels during MCAO. (Left) Rhodamine 6G labeling revealed that proximal occlusion caused a rapid and marked margination of leukocytes in venules downstream of the MCA (upper), which persisted and continued developing after recanalization (lower). Scale bar, 100 µm. (Right) tMCAO eventually led to secondary occlusion of postcapillary venules characterized by accumulation of platelets and fibrin(ogen). Scale bar, 50 µm. A, arteriole; V, venule. Adapted from Desilles et al49 with permission.
Prevention of inflammatory bleeding by platelets can occur independent of G protein–coupled receptors and GPIIbIIIa. (A) Representative images of cutaneous rpa spots showing that, unlike thrombocytopenic mice (<3% platelets), mice treated with a blocking F(ab')2 fragment antibody to GPIIb/IIIa did not develop bleeding in the inflamed skin. Adapted from Deppermann et al.33 (B) Representative images of mouse mammary tumor showing that, unlike thrombocytopenic mice (<3% platelets), mice with a genetic deficiency in β 3 integrins (β3−/−) did not develop tumor bleeding. Adapted from Ho-Tin-Noé et al49 with permission. (C) Transgenic hIL-4Rα/GPIbα mice were immunodepleted for platelets and transfused or not with control wild-type platelets or with platelets isolated from clopidogrel and aspirin-treated PAR4−/− mice. Those mice were then challenged in the skin with the rpA or in the lungs with LPS, as indicated. Representative images of the rpa site (dashed outlines and arrows) and of the bronchoalveolar lavage fluid are shown. Remarkably, just like control wild-type platelets, clopidogrel- and aspirin-treated PAR4−/− platelets protected against inflammatory bleeding in the inflamed skin and lungs. Adapted from Boulaftali et al32 with permission. (D) Representative images showing brain sections from mice that were subjected to tMCAO. The photographs were taken 24 hours after reperfusion. Hemorrhagic transformation under the form of petechial bleeding developed in the ischemic hemisphere of thrombocytopenic mice (<3% platelets) and of mice treated with a blocking F(ab')2 fragment antibody to GPIIb/IIIa. (Upper) Adapted from Goerge et al.32 (Lower) Adapted from Kleinschnitz et al.48 (E) Intravital imaging of cortical microvessels during MCAO. (Left) Rhodamine 6G labeling revealed that proximal occlusion caused a rapid and marked margination of leukocytes in venules downstream of the MCA (upper), which persisted and continued developing after recanalization (lower). Scale bar, 100 µm. (Right) tMCAO eventually led to secondary occlusion of postcapillary venules characterized by accumulation of platelets and fibrin(ogen). Scale bar, 50 µm. A, arteriole; V, venule. Adapted from Desilles et al49 with permission.
Apparent redundancy or GPCRs and GPIIb/IIIa suggests that the mechanisms of inflammation-associated hemostasis differ from those that govern traumatic injury and arterial thrombosis. Nevertheless, care should be taken not to generalize these findings to all situations in which nontraumatic challenges to vascular integrity are associated with inflammatory responses. For example, pharmacological inhibition of GPIIb/IIIa resulted in hemorrhagic transformation similar to that induced with severe thrombocytopenia15 in the mouse brain after cerebral IR injury induced by transient occlusion of the middle cerebral artery (tMCAO)48 in mice (Figure 2D), which is associated with the formation of platelet/leukocyte aggregates in postcapillary venules in the MCA territory49,50 (Figure 2E). Accordingly, patients receiving dual antiplatelet therapy with aspirin and P2Y12 inhibitors are at greater risk for thrombolysis-related hemorrhagic transformation in acute ischemic stroke.51,52 Transient MCAO distinguishes itself from the other inflammatory models studied, in that it involves additional vascular damage from prolonged oxygen and glucose deprivation followed by hemodynamic stress induced by sudden recanalization. This combination likely results in more severe vascular damage that necessitates platelet aggregation. Transient MCAO also differs notably from the rpA reaction, in that platelet granule secretion is required to prevent bleeding.33 Further studies are warranted to address whether the requirement for platelet aggregation reflects on the extent of injury or the engagement of distinct protective mechanisms. It will be of particular importance to expand the types of injury modeled (eg, IR, allergic reactions, sepsis) to different organs to dissect the contribution of vascular bed affected from the type of challenge encountered.
Engagement of platelet receptors contributes to inflammatory hemostasis
In addition to GPCR and integrins, platelets possess a battery of primary adhesion receptors, the foremost among them being glycoprotein (GP)VI and GPIb-IX-V, which are their main receptors for collagen and von Willebrand factor (VWF),53 respectively.6 Because they lack the extracellular domain of GPIbα, hIL4Rα/GPIbα transgenic mice are commonly used to study the role of GPIb-IX-V. No bleeding was observed in the skin of hIL4Rα/GPIbα transgenic mice in the cutaneous rpA.15 Subcutaneous tumors also did not bleed after treatment with an inhibitor of the binding function of GPIbα23, and GPIbα deficiency did not induce hemorrhagic transformation after tMCAO in hIL4Rα/GPIbα transgenic mice or in mice treated with a blocking antibody to GPIbα.48,54 VWF deficiency was also not associated with bleeding in the cutaneous rpA,15 subcutaneous tumors,23,24 or tMCAO model.55 Thus, at least in the models and organs tested, inflammatory hemostasis is independent of the platelet GPIbα/VWF axis. Notably, although GPIbα or VWF deficiency did not induce brain hemorrhage in the tMCAO model, they both reduced infarct volume and improved functional outcome, suggesting that the protective action of platelets against inflammatory bleeding in the brain can nevertheless be dissociated from mechanisms driving thrombosis and potentially pro-inflammatory activities, thus offering exciting therapeutic possibilities.56
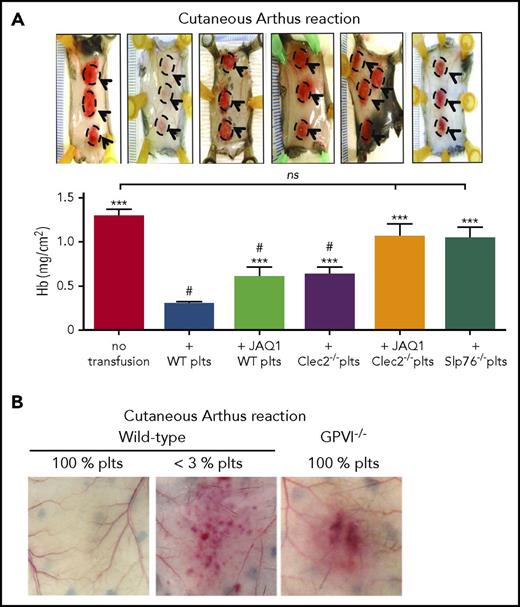
The role of the GPVI/collagen axis in inflammatory hemostasis was investigated together with that of the C-type lectin-like receptor 2 (CLEC-2), a platelet receptor for the glycoprotein podoplanin that was shown to provide partial functional redundancy with GPVI in models of hemostasis and thrombosis.57 GPVI and CLEC-2 are both members of the immunoreceptor tyrosine-based activation motif (ITAM) receptor family and share similar signaling pathways.58 Unlike GPVI, CLEC-2 is a hem(ITAM) receptor with only 1 tyrosine motif in its intracellular domain and differs from GPVI in signaling initiation.59-61 The adoptive platelet transfer model described here was used to demonstrate that GPVI or CLEC-2 deficiency reduced the capacity of platelets to rescue bleeding during the cutaneous rpA and LPS-induced lung inflammation (Figure 3A).32 Platelets with compound deficiency in GPVI and CLEC-2, or in SLP-76, a common effector of GPVI and CLEC-2,62 completely failed to support hemostasis in inflamed skin or lungs (Figure 3A). This suggested a critical role for ITAM signaling in inflammatory hemostasis. Yet because platelet counts were incompletely restored after transfusion,32 it is conceivable that relative thrombocytopenia exacerbated the effect of defects in adhesion or activation, thus sensitizing recipients to inflammatory bleeding in this study. The roles of GPVI and CLEC-2 have since been investigated in global GPVI- and platelet-selective CLEC-2-deficient mice, both of which have normal platelet counts. Skin bleeding in the cutaneous rpA model was confirmed in GPVI−/− mice34 (Figure 3B). Surprisingly, although platelet CLEC-2 protected against lung injury after intratracheal LPS instillation, it did not prevent lung bleeding.63 The discrepancy between these results and those obtained with the adoptive platelet transfer method32 stresses the need to confirm and further explore the roles of GPVI and CLEC-2 in inflammation and tumor-associated hemostasis in mice with normal platelet counts. If the capacity of thrombocytopenia to improve drug delivery to tumors22,24,64 can be reproduced by targeting GPVI or CLEC-2, this could potentiate the effects of chemotherapy without risk for major bleeding, as neither receptor is critical for classical hemostasis.57,58
GPVI and CLEC-2 contribution to the prevention of inflammatory bleeding by platelets. (A) Transgenic hIL-4Rα/GPIbα mice were immunodepleted for platelets and transfused or not with control wild-type platelets, or with platelets with a deficiency in GPVI (JAQ1 WT platelets), CLEC-2 (Clec2−/− platelets), GPVI and CLEC-2 (JAQ1 Clec2−/− platelets), or in SLP76 (Slp76−/− platelets). Mice were then challenged with the cutaneous reverse passive Arthus reaction rpA and compared for inflammatory bleeding at the reaction site. Representative images of challenged skin (reaction spots are outlined) are shown above the bar graphs, which represent the mean hemoglobin content at the reaction site. Unlike control wild-type platelets, platelets with a deficiency in GPVI or CLEC-2 only partially prevented inflammatory bleeding. Notably, platelets with a combined deficiency in GPVI and CLEC-2, or platelets lacking Slp76, a signaling adapter protein downstream of GPVI and CLEC-2, did not prevent bleeding when transfused to thrombocytopenic hIL-4Rα/GPIbα–mice. Adapted from Boulaftali et al32 with permission. (B) Representative photographs showing that GPVI−/− mice with normal platelet count (GPVI−/− 100% platelets) are sensitized to rpa-induced skin bleeding. Adapted from Gros et al.34
GPVI and CLEC-2 contribution to the prevention of inflammatory bleeding by platelets. (A) Transgenic hIL-4Rα/GPIbα mice were immunodepleted for platelets and transfused or not with control wild-type platelets, or with platelets with a deficiency in GPVI (JAQ1 WT platelets), CLEC-2 (Clec2−/− platelets), GPVI and CLEC-2 (JAQ1 Clec2−/− platelets), or in SLP76 (Slp76−/− platelets). Mice were then challenged with the cutaneous reverse passive Arthus reaction rpA and compared for inflammatory bleeding at the reaction site. Representative images of challenged skin (reaction spots are outlined) are shown above the bar graphs, which represent the mean hemoglobin content at the reaction site. Unlike control wild-type platelets, platelets with a deficiency in GPVI or CLEC-2 only partially prevented inflammatory bleeding. Notably, platelets with a combined deficiency in GPVI and CLEC-2, or platelets lacking Slp76, a signaling adapter protein downstream of GPVI and CLEC-2, did not prevent bleeding when transfused to thrombocytopenic hIL-4Rα/GPIbα–mice. Adapted from Boulaftali et al32 with permission. (B) Representative photographs showing that GPVI−/− mice with normal platelet count (GPVI−/− 100% platelets) are sensitized to rpa-induced skin bleeding. Adapted from Gros et al.34
It is noteworthy that in the tMCAO model, where GPIIb/IIIa is required to prevent cerebral hemorrhage, bleeding complications were not observed in mice treated with a GPVI-depleting antibody,48 again suggesting that the functional hierarchy between platelet adhesion receptors in the prevention of inflammatory bleeding may be challenge and/or vascular bed dependent.
Platelets counter vascular damage associated with leukocyte trafficking
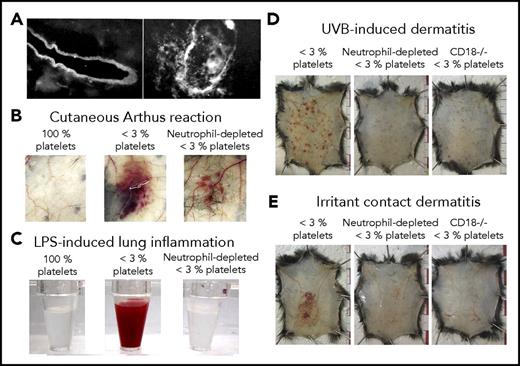
As outlined here, platelets prevent bleeding independent of GPIIb/IIIa-mediated aggregation in at least 4 inflammation-associated models: IgG complex-mediated cutaneous rpA reaction15,32-34 (Figures 2-4), LPS-induced lung inflammation32,33 (Figures 2-4), syngeneic tumors grafted subcutaneously23,24,43 (Figure 2), and immunization with ovalbumin/complete Freunds’ Adjuvant.44 Evidence that GPVI and CLEC-2, the only ITAM receptors present on mouse platelets (human platelets further express the IgG Fc receptors FcγRIIA), could be responsible for engaging platelets has been provided in 3 of these models32,34,44 ; their roles in the protection of tumor vessels remain to be investigated. Historic EM studies provide clues about how ITAM receptors may operate to ensure hemostasis in inflamed organs without triggering or requiring platelet aggregation. Various studies, including those by Majno and Palade,5,65 converged on the conclusion that the basement membrane retains blood cells and some of the larger molecules and particles such as lipoproteins and chylomicrons on opening of endothelial junctions in inflamed postcapillary venules. In a 1977 review, Ryan and Majno suggested that although interruptions in the endothelial lining suffice for plasma fluid and small molecules to leak out of blood vessels, the underlying basement membrane is “surprisingly tough,” serving as a “coarse filter.”66 Accordingly, subcutaneous injections of the pro-permeability factors histamine and vascular endothelial growth factor receptor-A, which do not disrupt the basement membrane, did not cause bleeding in thrombocytopenic mice.20,43 In 1966, a few years after demonstrating the central role played by neutrophils in the initiation of the cutaneous rpA,67 Cochrane described the requirement for neutrophil extravasation for degradation of the basement membrane in 2 IgG-mediated immune reactions, including the rpA68 (Figure 4A). The same study established a role of neutrophils in the degradation of the basement membrane by showing that they contained agents injurious to the basement membrane and that inflammation-induced basement membrane alterations were prevented by their depletion. More recent studies have shown that neutrophil transmigration takes place in specialized “permissive” regions of the vascular basement membrane, and that neutrophil-derived proteases cause mechanical alterations in such “low-expressing regions,” where components of the basement membrane are expressed at a lower density.69,70 Accordingly, if mice lack or are deprived of their capacity to recruit neutrophils, residual inflammation no longer triggers bleeding during the cutaneous rpA20,34 (Figure 4B), LPS-induced lung inflammation20 (Figure 4C), or glomerulonephritis,25 or in models of ultraviolet B- and irritant contact-induced dermatitis20 (Figure 4D). This role may not be exclusive to neutrophils, as tumor bleeding in thrombocytopenic mice involves both infiltrating neutrophils and macrophages,43 and bleeding in draining lymph nodes after immunization also involves lymphocytes44 (Table 1).
Platelets are required to seal vascular injuries inflicted by infiltrating leukocytes. (A) Immunostaining of the basement membrane of skin vessels before (left) and after (right) neutrophil transmigration had occurred during the cutaneous Arthus reaction in a rabbit. Adapted from Cochrane and Aikin68 with permission. (B-C) Representative photographs showing the aspect of the skin after 4 hours of cutaneous reverse passive Arthus reaction (B) or of the bronchoalveolar lavage fluid collected after 24 hours of LPS-induced lung inflammation (C), in control mice (100% platelets) and in mice that were immunodepleted for platelets alone (<3% platelets) or for platelets and neutrophils (neutrophil-depleted <3% platelets). Note that in both inflammatory models, neutrophil depletion before the reaction led to the prevention of inflammatory bleeding. (B) Adapted from Gros et al34 with permission. (C) Adapted from Hillgruber et al20 with permission. (D-E) Representative photographs showing that thrombocytopenic mice depleted for neutrophils or with defective neutrophil recruitment to the inflamed skin because of a genetic deficiency beta2 integrin (CD18−/−) are protected toward ultraviolet B radiation- or irritant contact dermatitis-induced petechial skin bleeding. Adapted from Hillgruber et al20 with permission.
Platelets are required to seal vascular injuries inflicted by infiltrating leukocytes. (A) Immunostaining of the basement membrane of skin vessels before (left) and after (right) neutrophil transmigration had occurred during the cutaneous Arthus reaction in a rabbit. Adapted from Cochrane and Aikin68 with permission. (B-C) Representative photographs showing the aspect of the skin after 4 hours of cutaneous reverse passive Arthus reaction (B) or of the bronchoalveolar lavage fluid collected after 24 hours of LPS-induced lung inflammation (C), in control mice (100% platelets) and in mice that were immunodepleted for platelets alone (<3% platelets) or for platelets and neutrophils (neutrophil-depleted <3% platelets). Note that in both inflammatory models, neutrophil depletion before the reaction led to the prevention of inflammatory bleeding. (B) Adapted from Gros et al34 with permission. (C) Adapted from Hillgruber et al20 with permission. (D-E) Representative photographs showing that thrombocytopenic mice depleted for neutrophils or with defective neutrophil recruitment to the inflamed skin because of a genetic deficiency beta2 integrin (CD18−/−) are protected toward ultraviolet B radiation- or irritant contact dermatitis-induced petechial skin bleeding. Adapted from Hillgruber et al20 with permission.
Although these studies argue that leukocytes cause inflammatory bleeding, leukocyte emigration per se does not induce bleeding even in thrombocytopenia. Endothelial junctions are indeed designed to facilitate the active passage of leukocytes without passive leak of plasma or erythrocytes.66,71-73 IVM studies also reveal that not all vessels undergoing neutrophil infiltration bleed, whether in GPVI−/− or thrombocytopenic mice.34 It thus appears that platelets are needed only at sites where leukocyte emigration coexists with endothelial damage or extensive junctional opening or dysfunction. This is supported by a historic study by Hurley showing that intravenously injected carbon particles extravasate only during inflammation, and only from vessels that feature both neutrophils and gaps in the endothelium.71
Single platelets seal neutrophil-induced vascular breaches during inflammation
There are multiple ways by which platelets could counteract leukocyte-induced vascular injury. One apparent possibility would be to restrict leukocyte infiltration. Yet observations that platelets and leukocytes mutually support their recruitment to the inflamed vasculature of various organs74,75 and that platelet depletion is associated with a marked decrease in neutrophil infiltration in the cutaneous rpA reaction20,34,38 argue against this model. A second possibility is that platelets dampen neutrophil degranulation and histotoxic activities. Surprisingly, however, thrombocytopenic and GPVI−/− mice both showed reduced levels of neutrophil oxidative activity, as well as reduced release of neutrophil-derived MMP-9 and MPO in the IgG-mediated rpA reaction,34 arguing that platelets and GPVI stimulate rather than limit the tissue-damaging activities of neutrophils in this model. A third possibility would be that platelets play a 2-dimensional structural role in sealing vascular breaches generated by neutrophils, as suggested by Majno, Cotran, and others.
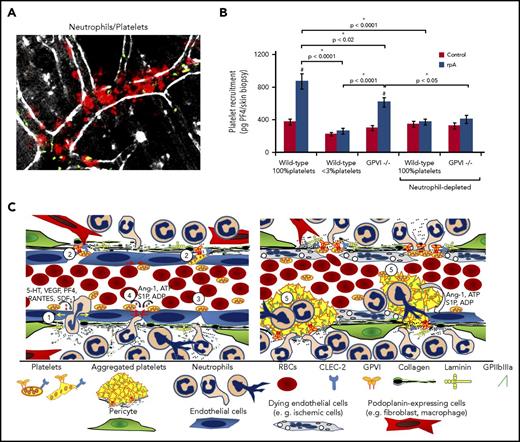
Analysis of the inflamed vasculature during the cutaneous rpA by IVM supports this model, revealing rapid recruitment of platelets to the vessel wall of postcapillary venules at the reaction site, where neutrophils also accumulate34 (Figure 5A). Individual platelets bound to adherent neutrophils were also frequently observed (Figure 5A). Platelet recruitment in the inflamed skin was reduced in GPVI−/− mice and suppressed by neutrophil depletion (Figure 5B). Similar results were obtained using microspheres coated with a chimeric form of GPVI (GPVI-Fc76 ) to detect the exposure of GPVI-binding sites. GPVIFc-coated microspheres adhered firmly to the vessel wall and to marginating neutrophils at the reaction site, and their recruitment was abolished by neutrophil depletion. These results confirmed that marginating neutrophils can recruit platelets by binding them directly,77 and further indicated that they unmask binding sites for platelets and GPVI on the wall of inflamed venules; staining of the endothelial lining revealed interruptions in areas of neutrophil extravasation (Figure 5A). As a receptor for collagen, other adhesive components of the basement membrane and extracellular matrix,78-81 and fibrin,82,83 GPVI could help platelets seal vascular breaches by mediating their adhesion to areas where the basement membrane is exposed and disrupted by neutrophils. GPVI is also a receptor for the extracellular matrix metalloproteinase inducer (EMMPRIN),84,85 a signaling receptor86 expressed by leukocytes, including neutrophils,87 hinting that GPVI/EMMPRIN interactions may trigger reciprocal signals in both platelets and neutrophils. Accordingly, GPVIFc-coated microspheres were also observed to bind to marginating neutrophils.34
How platelets repair vascular injuries inflicted by infiltrating neutrophils. (A) Early platelet recruitment to the vessel wall during the cutaneous rpA, reaction as investigated by intravital microscopy. Platelets and neutrophils were stained in vivo, using fluorescent antibodies to GPIbβ (green color) and Ly-6G (red color). Single platelets were seen firmly adhering to the vessel wall and to neutrophils in areas of marked neutrophil margination and transmigration, which occurred in postcapillary venules. Scale bar, 100 µm. (B) Comparison of early platelet accumulation at the rpa site between control and thrombocytopenic wild-type mice and GPVI−/− mice, as assessed by measurement of skin PF4 content. In wild-type mice, the rpA led to the recruitment of platelets, which was reduced in GPVI−/− mice. Note that platelet recruitment to the inflamed skin was prevented by neutrophil depletion. Adapted from Gros et al.34 (C) Schematic representation of how platelets can prevent bleeding from sites of neutrophil extravasation in postcapillary venules. (Left) Single platelets are recruited to the inflamed vessel walls through interactions with activated endothelial cells and neutrophils, and with the exposed basement membrane. Recruited platelets play a dual role by promoting edema formation (yellow arrows, 1) and amplifying neutrophil infiltration through the secretion of permeability factors and chemokines (eg, serotonin, vascular endothelial growth factor receptor, PF4, RANTES, SDF-1), while ensuring inflammatory hemostasis. Concomitantly with or subsequently to their pro-inflammatory effects, single platelets indeed prevent bleeding by filling gaps between endothelial cells and plugging breaches in the basement membrane caused by extravasating neutrophils (2). This protective action of platelets would notably involve interactions between GPVI and components of the exposed subendothelium (eg, laminin or collagen), and/or between CLEC-2 with podoplanin-expressing stromal cells in close proximity to blood vessels (eg, macrophages, fibroblasts). In addition to a mechanical action, platelets engaged by these pathways might also trigger vascoconstriction, bridge endothelial gaps (3), or promote closing of endothelial junctions (4) over holes in the basement membrane, through the secretion of antipermeability and endothelial cell survival factors (eg, S1P, angiopoietin-1, ADP, ATP). (Right) In case of more severe endothelial injury, as in models of cerebral ischemia-reperfusion, inflammatory hemostasis would rely on more classical mechanisms including GPIIb/IIIa-mediated platelet aggregation (5).
How platelets repair vascular injuries inflicted by infiltrating neutrophils. (A) Early platelet recruitment to the vessel wall during the cutaneous rpA, reaction as investigated by intravital microscopy. Platelets and neutrophils were stained in vivo, using fluorescent antibodies to GPIbβ (green color) and Ly-6G (red color). Single platelets were seen firmly adhering to the vessel wall and to neutrophils in areas of marked neutrophil margination and transmigration, which occurred in postcapillary venules. Scale bar, 100 µm. (B) Comparison of early platelet accumulation at the rpa site between control and thrombocytopenic wild-type mice and GPVI−/− mice, as assessed by measurement of skin PF4 content. In wild-type mice, the rpA led to the recruitment of platelets, which was reduced in GPVI−/− mice. Note that platelet recruitment to the inflamed skin was prevented by neutrophil depletion. Adapted from Gros et al.34 (C) Schematic representation of how platelets can prevent bleeding from sites of neutrophil extravasation in postcapillary venules. (Left) Single platelets are recruited to the inflamed vessel walls through interactions with activated endothelial cells and neutrophils, and with the exposed basement membrane. Recruited platelets play a dual role by promoting edema formation (yellow arrows, 1) and amplifying neutrophil infiltration through the secretion of permeability factors and chemokines (eg, serotonin, vascular endothelial growth factor receptor, PF4, RANTES, SDF-1), while ensuring inflammatory hemostasis. Concomitantly with or subsequently to their pro-inflammatory effects, single platelets indeed prevent bleeding by filling gaps between endothelial cells and plugging breaches in the basement membrane caused by extravasating neutrophils (2). This protective action of platelets would notably involve interactions between GPVI and components of the exposed subendothelium (eg, laminin or collagen), and/or between CLEC-2 with podoplanin-expressing stromal cells in close proximity to blood vessels (eg, macrophages, fibroblasts). In addition to a mechanical action, platelets engaged by these pathways might also trigger vascoconstriction, bridge endothelial gaps (3), or promote closing of endothelial junctions (4) over holes in the basement membrane, through the secretion of antipermeability and endothelial cell survival factors (eg, S1P, angiopoietin-1, ADP, ATP). (Right) In case of more severe endothelial injury, as in models of cerebral ischemia-reperfusion, inflammatory hemostasis would rely on more classical mechanisms including GPIIb/IIIa-mediated platelet aggregation (5).
The CLEC-2 ligand podoplanin may serve a similar and partially redundant role to GPVI ligands in recruiting platelets both to leukocytes and to tissues where it is prevalent, such as tumors and brain. Although normally absent from the blood endothelium or mural cells,88 podoplanin expression can be observed in the vicinity of blood vessels and becomes more widespread during inflammation,89 as observed in skin and the vessel wall of the inferior vena cava.90,91 Even if not in the first line of defense, podoplanin-expressing cells surrounding blood vessels may recruit platelets through CLEC-2 in regions of basement membrane rupture, or where lower basement membrane density could favor cell interactions (Figure 5C). Supporting this model, lymphocyte trafficking in lymph nodes was shown to cause bleeding from high endothelial venules in mice lacking CLEC-2 on platelets or podoplanin on underlying fibroblastic reticular cells.44
GPVI and CLEC-2 are not only adhesion receptors but also signaling receptors, and the inability of platelets lacking SLP-76 to rescue bleeding in inflamed lungs and skin in the adoptive platelet transfer model32 (Figure 3A) supports a role for ITAM signaling in inflammation-associated hemostasis. Moreover, in the cutaneous rpA, treatment of wild-type platelets with high doses of inhibitors of the spleen tyrosine kinase (Syk) and Bruton’s tyrosine kinase, 2 transducers of GPVI and CLEC-2 signaling, impaired their ability to prevent bleeding when transfused to GPVI−/− mice without affecting their recruitment to the reaction site. Pointing to a possible dominant role for Syk, platelets treated with a Bruton’s tyrosine kinase-inhibitor alone maintained vascular integrity in inflamed lungs and skin,92 despite losing most aggregation capacity in vitro. Syk plays an essential role in platelet filopodia formation, but not in GPVI potentiation of platelet activation by thrombin.93 Interestingly, the hemITAM motif of CLEC-2 is required for blood-lymph separation during embryogenesis, but not for the role of CLEC-2 signaling in thrombosis.94 These observations are concordant with other observations suggesting that aggregation is dispensable for inflammation-associated hemostasis, and instead argue a role for ITAM signaling in platelet spreading in this context.
In light of the observations that inflammatory hemostasis involves single platelets, the requirement for CLEC-2 and GPVI argues that these receptors are either not sufficient or actively prevented from triggering platelet aggregation when binding to their ligands in vivo in the absence of thrombin or other strong stimuli. Platelet shape change and secretion can indeed be triggered independent of aggregation, notably in response to collagen.95,96 In solid tumors, platelets pretreated with thrombin and emptied of their content before transfusion to thrombocytopenic mice failed to confer the protection against tumor bleeding observed with equivalent numbers of wild-type platelets, suggesting a potential role for paracrine interactions between platelets and endothelial cells mediated by platelet-derived mediators.23 Platelets contain abundant amounts of the bioactive endothelial barrier protective lipid sphingosine-1-phosphate (S1P),97 and mice deficient in S1P in plasma and circulating cells are prone to lymph node bleeding during immunization.44 Selective deficiency in S1P production in megakaryocytes impaired platelet spreading on fibrinogen and deprived platelets of their capacity to promote endothelial barrier function ex vivo.21 However, it did not reproduce lymph node bleeding after immunization, as observed with combined S1P deficiency in plasma and all circulating cells, nor did it impair inflammation-associated hemostasis in lung or skin.21 Accordingly, mice with genetic deficiency in both platelet α- and dense-granule secretion (Unc13d−/−/Nbeal2−/−) also did not bleed in the inflamed skin and lungs.33 Although this appears to suggest that platelet granule content is dispensable for the prevention of inflammatory bleeding in these organs, it is important to consider that reduced neutrophil infiltration could also contribute to the protection of Unc13d−/−/Nbeal2−/− mice from inflammatory bleeding, as platelets substantially enhance neutrophil recruitment through the release of chemokines stored in their granules.38,39,98 Moreover, as deficiency was not restricted to platelets, decreased neutrophil granule secretion could also reduce inflammatory damage in this model. In accordance with the need of GPIIb/IIIa and platelet aggregation to secure hemostasis in tMCAO,33,48-50 Unc13d−/−/Nbeal2−/− mice bled after tMCAO, although bleeding was not observed when secretion deficiency was limited to α- or dense granules,99,100 hinting at functional redundancy between α- and dense granule-derived factors in this context (Figure 5C).
These observations argue that the principal hemostatic role of platelets during inflammation may be to form a physical barrier created by single platelets or sheets of platelets, depending on the level of endothelial activation and damage, and that signaling is required to optimize spreading, but not aggregation. Platelet secretion and aggregation may be enlisted only when the injury and challenge to barrier integrity is sufficiently severe to engage the coagulation cascade and classical hemostatic mechanisms, and the respective role for adhesion receptors may vary with challenge, organ, and vessel subtype (Table 1, Figure 5C).
Clinical relevance of inflammation-associated hemostasis
If inflammatory hemostasis is both important and mechanistically distinct from classical hemostasis, why is it not more clinically visible? This may be because it can operate at very low platelet counts, is resistant to current antiplatelet therapy, and is sustained by partially redundant receptors. A useful analogy may come from coagulation. Without tissue factor, embryos do not survive development.101 If tissue factor deficiency is induced postnatally, lethal bleeding ensues.101 In contrast, mice with mutations in the intrinsic pathway survive development without spontaneous bleeding.101 Blocking the intrinsic pathway provides admirable anticoagulant effects with limited bleeding complications because the tissue factor-dependent extrinsic pathway secures a basal level of coagulation activation.102,103 Analogous to the extrinsic pathway, inflammation-associated hemostasis may be what helps to limit the bleeding risk for current antithrombotic therapies to the context of severe vascular or ischemic injury.104 Conversely, and in contrast to the extrinsic pathway, however, consequences of deficiencies in inflammation-associated hemostasis are not severe, as it can be bypassed for classical hemostasis by various pathways leading to aggregation.93 GPVI deficiency and thrombocytopenia without superimposed traumatic challenge suggest the clinical manifestations of deficiencies in inflammation-associated hemostasis. Both are associated with cutaneous and other relatively benign bleeding complications.
Does inflammation-associated hemostasis offer therapeutic opportunities or risks? There may be risks associated with emerging strategies targeting GPVI combined with conventional platelet antagonists. Opportunities may lie in the identification of patients at risk of bleeding with conventional therapies, and in the use of anti-inflammatory therapies to prevent bleeding in patients with thrombocytopenia.
Conclusions
The ability of platelets to prevent bleeding independent of aggregation has gained attention in recent years because it helps explain why selective targeting of mechanisms driving platelet aggregation can efficiently prevent occlusive thrombus formation without a substantial risk of bleeding under homeostasis.104 Although the functional hierarchy between platelet receptors in this form of hemostasis may vary with degree and type of challenge and vascular bed, a common theme is emerging where single platelets seal leukocyte-induced vascular breaches by covering the injured basement membrane, either by filling endothelial gaps or by bridging endothelial junctions (Figure 5C). This model is largely supported by historical observations and may have been considered as a component of hemostasis in the past. Recent developments in mouse genetics and microscopy have propelled a rediscovery of this phenomenon and clearly defined it as a distinct process that is triggered by endothelial activation and dysfunction, as observed in tumors and inflamed organs, and in which platelet ITAM receptors could play a predominant role.
Acknowledgments
This work was supported by grants from Force Hémato, INCA, and La Fondation Arc (PJA 20151203107) (B.H.-T.-N.); by La Fondation pour la Recherche Medicale (FRM ARF20140129191), Marie Curie Action (Reintegration Grant 708973), and DHU Fire 012 (Y.B.); and the French National Research Agency (ANR-10-MIDI-0003) and the Leducq Foundation (SphingoNet) (E.C.).
Authorship
Contribution: Y.B., E.C., and B.H.-T.-N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benoît Ho-Tin-Noé, INSERM U1148, Hôpital Bichat, 46 rue Henri Huchard, 75877 Paris Cedex 18, France; e-mail: benoit.ho-tin-noe@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal