In this issue of Blood, Green et al identified an innovative and promising pretargeted radioimmunotherapy (RIT) approach for the treatment of non-Hodgkin lymphoma (NHL) and multiple myeloma. Pretargeted RIT of B-cell malignancies with a CD38 bispecific monoclonal antibody (mAb) is a novel approach, and the results achieved in preclinical models are very impressive. CD38 is an excellent target because it has high density and stable expression in multiple myeloma and NHL.1
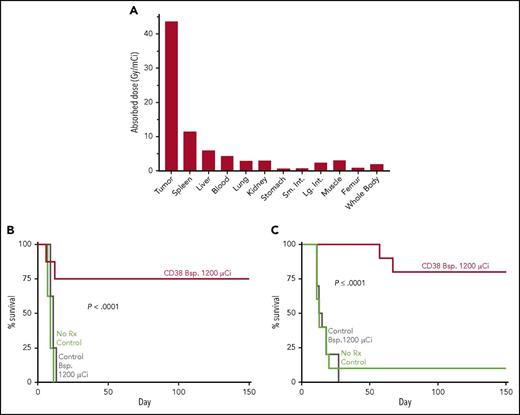
A novel bispecific antibody against CD38 (CD38 Bsp.) and DOTA-biotin eradicates NHL and multiple myeloma (MM) tumors in murine models. In this pretargeted approach, the CD38 or CD20 control Bsp. was injected IV first. After tumor targeting and clearance from the circulation, 90Y-DOTA-biotin was injected IV and pharmacokinetic studies were carried out to calculate tumor and normal tissue dosimetry, and survival of mice was monitored. (A) Tumor and normal tissue dosimetry in CD38 Bsp. Namalwa NHL model. (B) Survival of mice bearing Namalwa NHL tumors administered CD38 or CD20 control Bsp. and 90Y-DOTA-biotin. (C) Survival of mice bearing H929 MM tumors and administered CD38 or CD20 control Bsp. and 90Y-DOTA-biotin. The figure has been adapted from Figures 4, 5B, and 6B in the article by Green et al that begins on page 611.
A novel bispecific antibody against CD38 (CD38 Bsp.) and DOTA-biotin eradicates NHL and multiple myeloma (MM) tumors in murine models. In this pretargeted approach, the CD38 or CD20 control Bsp. was injected IV first. After tumor targeting and clearance from the circulation, 90Y-DOTA-biotin was injected IV and pharmacokinetic studies were carried out to calculate tumor and normal tissue dosimetry, and survival of mice was monitored. (A) Tumor and normal tissue dosimetry in CD38 Bsp. Namalwa NHL model. (B) Survival of mice bearing Namalwa NHL tumors administered CD38 or CD20 control Bsp. and 90Y-DOTA-biotin. (C) Survival of mice bearing H929 MM tumors and administered CD38 or CD20 control Bsp. and 90Y-DOTA-biotin. The figure has been adapted from Figures 4, 5B, and 6B in the article by Green et al that begins on page 611.
A major limitation for RIT of NHL with directly radiolabeled anti-CD20 mAb is bone marrow toxicity due to the long circulation time.2 Efforts to use radiolabeled mAb fragments for RIT have not been successful because of their rapid elimination from the circulation and low tumor uptake. There have been several pretargeted RIT strategies investigated to overcome these limitations, involving administration of an unlabeled targeting molecule designed to localize rapidly and preferentially in tumor sites, followed by intravenous injection of a clearing agent to remove the nontumor bound targeting molecule from the circulation to reduce the radiation absorbed dose to the bone marrow, followed by administration of a radiolabeled small molecule that binds to the targeting molecule localized in tumors. One pretargeting strategy used fusion constructs consisting of single chain antibodies that bind to a tumor antigen linked to streptavidin (SA), a clearing agent, and radiolabeled biotin.3 This approach was effective in an animal model of lymphoma using anti-CD20 single chain antibody fusion protein linked to SA and 90Y-labeled biotin.4 The anti-CD20 fusion protein-SA and 90Y-labeled biotin produced encouraging therapeutic results in a pilot trial in patients with NHL.5 However, the high immunogenicity of SA has been of concern for clinical trials because the immune response would preclude administration of multiple doses, and binding to endogenous biotin would limit the dose delivered to tumors. Additional investigations in lymphoma models have included other bispecific mAbs for localization of a radiolabeled hapten-peptide6 and a recombinant fusion protein using 2 anti-CD20 Fabs and 1 anti-hapten Fab.7
Green et al describe the efficacy in preclinical models of B-cell lymphoma and multiple myeloma of a CD38 bispecific antibody that binds to CD38, and a complex of the chelating agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) linked to biotin labeled with the β-particle emitter 90Y (90Y-DOTA-biotin). The CD38 bispecific antibody had excellent tumor targeting, and the subsequent administration of 90Y-DOTA-biotin produced a radiation absorbed tumor dose in the NHL model of 43.8 Gy/mCi, with tumor-to-normal organ dose ratios of 7:1 for liver, 15:1 for lung and kidneys, and 10:1 for blood (see figure panel A). In murine therapy studies, CD38 bispecific mAb and 90Y-DOTA-biotin produced 75% to 80% long-term survival in the B-cell lymphoma and multiple myeloma models (see figure panels B and C). The efficacy of the CD38 bispecific mAb pretargeting RIT was equal or superior to CD38-SA pretargeted RIT and was proportional to the radionuclide dose administered. The high efficacy of the CD38 bispecific mAb and 90Y-DOTA-biotin pretargeting combination indicates it is an attractive approach for clinical translation that may benefit patients with unresponsive, high-risk disease, because treatment refractory multiple myeloma and NHL typically retain radiation sensitivity.1 The group of investigators at the Fred Hutchinson Cancer Research Center are also developing bispecific fusion constructs for α-particle pretargeted RIT,8 which may be more effective against minimal residual disease and early metastatic disease because of the higher linear energy transfer and relative biological effectiveness of α-particle emitters as compared with β-particle emitters. The results of future clinical trials of CD38 bispecific pretargeted RIT in unresponsive NHL and multiple myeloma patients are anxiously awaited.
Editor’s note: Oliver Press, senior author of the article by Green et al, died of cancer on 29 September 2017. His work helped revolutionize therapy for B-cell malignancies. He was a consummate mentor for many trainees and was posthumously awarded a Mentor Award at the 2017 Annual Meeting of the American Society of Hematology.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal