Key Points
Duvelisib, an oral dual inhibitor of PI3K-δ and γ, is clinically and pharmacodynamically active across a range of hematologic malignancies.
75 mg twice daily was determined to be the MTD, with 25 mg twice daily selected for further evaluation in phase 2 and 3 studies.
Abstract
Duvelisib is an oral dual inhibitor of phosphoinositide 3-kinase-δ (PI3K-δ) and PI3K-γ in late-stage clinical development for hematologic malignancy treatment. This phase 1 study evaluated maximum tolerated dose (MTD), pharmacokinetics, pharmacodynamics (PD), efficacy, and safety of duvelisib in 210 patients with advanced hematologic malignancies. In the dose escalation phase (n = 31), duvelisib 8 to 100 mg twice daily was administered, with MTD determined as 75 mg twice daily. In the expansion phase (n = 179), patients with indolent non-Hodgkin lymphoma (iNHL), chronic lymphocytic leukemia (CLL), or T-cell lymphoma (TCL) were treated with 25 or 75 mg duvelisib twice daily continuously. Single-dose duvelisib was rapidly absorbed (time to maximum concentration, 1-2 hours), with a half-life of 5.2 to 10.9 hours. PD results showed inhibition of phospho-AKT (S473) in CLL tumor cells following a single dose and near-complete inhibition of CLL proliferation (Ki-67) by cycle 2. Clinical responses were seen across a range of doses and disease subtypes: iNHL overall response rate, 58% (n = 31) with 6 complete responses (CRs); relapsed/refractory CLL, 56% (n = 55) with 1 CR; peripheral TCL, 50% (n = 16) with 3 CR; and cutaneous TCL, 32% (n = 19). Median time to response was ∼1.8 months. Severe (grade ≥3) adverse events occurred in 84% of patients: neutropenia (32%), alanine transaminase increase (20%), aspartate transaminase increase (15%), anemia and thrombocytopenia (each 14%), diarrhea (11%), and pneumonia (10%). These data support further investigation of duvelisib in phase 2 and 3 studies. This trial was registered at clinicaltrials.gov as #NCT01476657.
Introduction
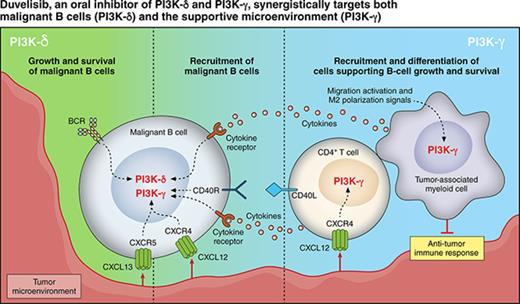
Duvelisib (also known as IPI-145)1 is an oral dual inhibitor of phosphoinositide-3-kinase-δ (PI3K-δ) and PI3K-γ. PI3K-δ is constitutively expressed in hematologic malignancies, and its inhibition has been shown to reduce the proliferation of various hematologic tumor cells while allowing normal immune cell survival.2-7 PI3K-δ inhibition directly targets proliferation and survival of malignant B-cell leukemia and lymphoma cells, whereas PI3K-γ inhibition reduces the differentiation and migration of key support cells in the tumor microenvironment, such as CD4+ T cells and M2 tumor-associated macrophages, which sustain leukemia and lymphoma cells in a protective niche.8-10
Given distinct and independent roles for PI3K-δ and PI3K-γ in supporting tumor growth and survival, their dual inhibition represents a broader mechanistic approach and may provide greater benefit than inhibiting just 1 isoform alone. This concept was demonstrated preclinically in a DoHH2 xenograft model of human transformed follicular lymphoma in which tumor growth inhibition was significantly greater in duvelisib-treated mice compared with those treated with a PI3K-δ–selective inhibitor11 and supports the rationale for advancing investigation of duvelisib as a therapy for hematologic malignancy patients.
Here we report the results from the first clinical study of duvelisib in patients with advanced hematologic malignancies, including maximum tolerated dose (MTD), pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety across numerous hematologic malignancies. Based upon these phase 1 data, duvelisib is being evaluated in phase 2 and 3 studies for the treatment of advanced hematologic malignancies, including indolent non-Hodgkin lymphoma (iNHL) and chronic lymphocytic leukemia (CLL).
Patients and methods
Study design and treatment
Study IPI-145-02 was a phase 1, open-label, dose-escalation study in patients with advanced hematologic malignancies. Duvelisib was administered as oral capsules twice daily continuously in 28-day cycles until disease progression or unacceptable toxicity. Dose interruptions and dose reductions (up to 2) were allowed in the event of duvelisib-related toxicities. Clinic visits occurred weekly during the first 3 treatment cycles, once every 2 weeks during cycles (C) 4 and 5, monthly during C6 through C19, and once every third cycle thereafter. The MTD was established in the dose escalation phase (DEP), which included patients with various types of advanced hematologic malignancies (see “Patients and eligibility criteria”). Dose escalation was executed in a standard 3+3 design, based on the occurrence of dose-limiting toxicities (DLTs) during C1. A DLT was defined as death, treatment-related grade 4 hematologic toxicity of >7 days' duration, or treatment-related nonhematologic ≥grade 3 toxicity. Doses in the DEP ranged from 8 to 100 mg twice daily. All patients who received at least 75% of their prescribed C1 doses or had a DLT during C1 were considered evaluable. The MTD was defined as the dose level below the dose at which >33% of evaluable patients experienced a DLT.
Based on safety, PK/PD, and efficacy results from the DEP, expansion cohorts at 25 mg twice daily were enrolled before reaching the MTD at 75 mg twice daily. Once the MTD was established, expansion cohorts at 75 mg twice daily were also opened.
Based upon known opportunistic infection risks in these heavily pretreated patient populations and safety events noted in this study, the protocol was proactively amended in March 2013 to require mandatory Pneumocystis prophylaxis for all patients, and in September 2014 to recommend herpes simplex virus prophylaxis. The original protocol included additional prophylaxis per institutional guidance or investigator discretion for cytomegalovirus and herpes zoster virus infection.
All patients signed an informed consent before undergoing any study procedure. The protocol was approved by investigative site institutional review boards, and the study was conducted per local and federal regulations and the Declaration of Helsinki.
Patients and eligibility criteria
Patients enrolled in the DEP were ≥18 years of age with a life expectancy >3 months and a diagnosis of advanced hematologic malignancy. Patients were required to have progressed on or been refractory to or intolerant of established therapy. Exclusion criteria included previous exposure to PI3K inhibitor(s); a diagnosis of overt leptomeningeal leukemia or central nervous system lymphoma; ongoing high-dose immunosuppression for chronic conditions; inadequate hepatic function, chronic hepatitis, or other chronic liver disease; inadequate bone marrow function (absolute neutrophil count <750 cells/mm3, platelets <75 000/mm3, and hemoglobin <8 g/dL without transfusion or cytokine support for >2 weeks before initiating study treatment); inadequate renal function; HIV infection; history of alcohol abuse; and pregnancy/lactation.
Enrollment criteria for the expansion phase were similar to the DEP, except that patients who previously received another PI3K inhibitor were eligible, and the exclusion for inadequate bone marrow function was removed. Eligible disease subtypes for the expansion phase included relapsed/refractory (RR) and treatment-naïve (TN) CLL, relapsed iNHL, and cutaneous and peripheral T-cell lymphoma (TCL). TN CLL patients were required to be ≥65 years of age and/or have a 17p deletion and/or TP53 mutation. Additional cohorts included patients with acute myeloid leukemia, myelodysplastic syndrome, myeloproliferative neoplasms, acute lymphoblastic T-cell leukemia/lymphoma, and acute lymphoblastic B-cell leukemia.
Pharmacokinetic methods
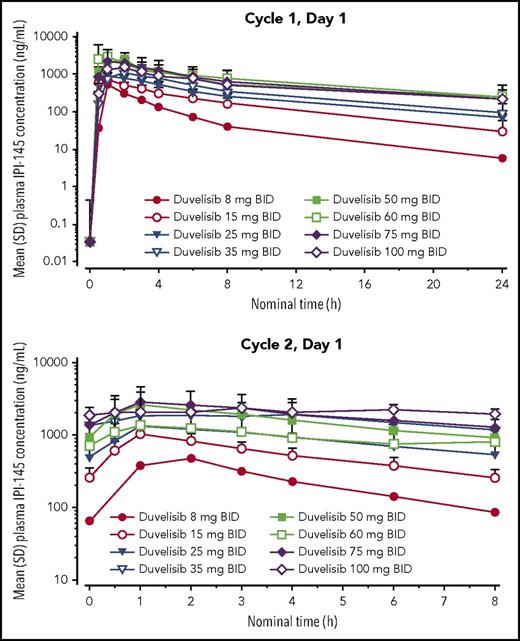
Plasma samples for PK analyses were obtained from patients in both phases predose and 0.5, 1, 2, 3, 4, 6, 8, and 24 (C1 only) hours after the morning dose of duvelisib on day 1 (D1) of C1 and C2 (Figure 1). Plasma samples were analyzed using a validated liquid chromatography with tandem mass spectrometry method (lower limit of quantitation of the assay = 1.00 ng/mL for both duvelisib and IPI-656, the major metabolite). IPI-656 is derived from oxidization of duvelisib and is inactive on PI3K-δ and PI3K-γ (Janid Ali, unpublished data, 10 May 2011). The PK analysis set included all patients with sufficient data to determine the PK parameters of duvelisib or IPI-656 following the first dose of duvelisib (supplemental Tables 1-3, available on the Blood Web site). PK parameters were derived using noncompartmental methods and included maximum plasma concentration (Cmax), time to Cmax, terminal elimination half-life, and area under the time-concentration curve (AUC).
Mean duvelisib plasma concentrations following single-dose (cycle 1, day 1) and multiple-dose (cycle 2, day 1) administration. BID, twice daily; SD, standard deviation.
Mean duvelisib plasma concentrations following single-dose (cycle 1, day 1) and multiple-dose (cycle 2, day 1) administration. BID, twice daily; SD, standard deviation.
Pharmacodynamic methods
Blood samples for PD analyses were collected in both the DEP and the expansion phase. To assess phospho-AKT (p-AKT) inhibition, whole blood samples were collected predose on C1D1 and at 1 and 24 hours after a single dose. CLL cells were evaluated by phospho-flow cytometry after coimmunostaining for CD19+, CD3+, CD5+, and p-AKT Ser473+. Validation of the assay was performed with whole blood samples from normal healthy donors that were treated with duvelisib ex vivo. Precision and accuracy was determined to have a coefficient of variation <15%, and stability of samples was satisfactory for 48 hours. The percentage of positive CD19+, CD3−, and CD5+ cells in the lymphocyte size gate that were p-AKT Ser473+ was determined.
Changes in the cell proliferation marker Ki67 were evaluated by flow cytometry using whole blood samples collected predose on C1D1 and C2D1 from CLL patients. The percentage of positive CD19+, CD3−, and CD5+ cells in the lymphocyte size gate that were Ki67+ was determined.
Using serum collected from iNHL or CLL patients, multiplex panels of cytokines, chemokines, and matrix metalloproteinases (72 total analytes) associated with the B-cell malignancy tumor microenvironment12 were evaluated using Luminex xMAP technology (Luminex Corp., Austin, TX). The PD analysis set consisted of patients with iNHL and CLL with a C1D1 predose sample and at least 1 other sample (C1D8, C2D1, C3D1, or C5D1).
Efficacy methods
Disease response (complete response [CR], partial response, stable disease or progression) was determined by the investigator per disease-specific response criteria on D1 of C3, C5, and C7; every third cycle from C10 through C19; and every sixth cycle thereafter using computed tomography scans and positron emission tomography, where indicated. A bone marrow biopsy and/or aspiration was performed to confirm a radiographic CR. Efficacy (response) is presented by disease subtype.
Safety methods
Adverse event (AE) monitoring and laboratory assessments were performed at all clinic visits. Severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. AE relationship to duvelisib treatment was based on investigator assessment. Safety summaries include all patients who received at least 1 dose of duvelisib.
Statistical methods
Descriptive statistics, including frequency and percentage for categorical end points, are presented. For the overall response rate (ORR), the Clopper-Pearson 95% confidence interval (CI) is provided. Statistical analyses comparing dose groups were not performed, and expansion cohort sample sizes were not prespecified. Analyses of PD data included 2-sample t test comparisons of C1D1 values compared with values from healthy donor samples (serum purchased from BioreclamationIVT, Hicksville, NY); and paired t tests of the change from C1D1 to subsequent on-treatment time points. These analyses were evaluated at an α of 0.05 after Bonferroni correction for multiple comparisons.
Results
Patient demographic characteristics, disposition, and treatment
Across the 210 patients who received duvelisib, the median age was 67 years (range, 25-86), and most were white (88%) and male (63%). Nearly all patients (93%) had an Eastern Cooperative Oncology Group Performance Status score <2 at baseline, and 63% had received ≥3 prior systemic anticancer regimens. Of the disease subtypes enrolled, most patients had RR CLL (n = 55; includes 5 with small lymphocytic lymphoma), followed by TCL (n = 35), iNHL (n = 31), aggressive NHL (n = 26), and TN CLL (n = 18) (Table 1).
Patient demographics and disease history
| Demographic parameter . | All patients (N = 210) . |
|---|---|
| Age (y), median (range) | 66.5 (25-86) |
| Race, white, n (%) | 185 (88.1) |
| Sex, male, n (%) | 132 (62.9) |
| ECOG score, 0/1/2, % | 28.1/65.2/6.7 |
| Number of prior systemic therapies, median (range) | 3.0 (0-11) |
| ≥3 prior systemic regimens, n (%) | 133 (63.3) |
| Current diagnosis at enrollment, n (%) | |
| iNHL | 31 (14.8) |
| FL | 24 (11.4) |
| iNHL (not otherwise specified) | 1 (0.5) |
| MZL | 2 (1.0) |
| WM/LPL | 4 (1.9) |
| CLL/SLL (RR) | 55 (26.2) |
| CLL | 50 (23.8) |
| SLL | 5 (2.4) |
| CLL (TN) | 18 (8.6) |
| T-cell NHL | 35 (16.7) |
| Cutaneous TCL | 19 (9.0) |
| Peripheral TCL | 16 (7.6) |
| Other diagnoses | 71 (33.8) |
| aNHL | 26 (12.4) |
| MCL | 10 (4.8) |
| AML | 6 (2.9) |
| MM | 3 (1.4) |
| HL | 3 (1.4) |
| Myelofibrosis | 5 (2.4) |
| MDS | 6 (2.9) |
| T-cell ALL | 5 (2.4) |
| B-cell ALL | 4 (1.9) |
| LGL | 3 (1.4) |
| Demographic parameter . | All patients (N = 210) . |
|---|---|
| Age (y), median (range) | 66.5 (25-86) |
| Race, white, n (%) | 185 (88.1) |
| Sex, male, n (%) | 132 (62.9) |
| ECOG score, 0/1/2, % | 28.1/65.2/6.7 |
| Number of prior systemic therapies, median (range) | 3.0 (0-11) |
| ≥3 prior systemic regimens, n (%) | 133 (63.3) |
| Current diagnosis at enrollment, n (%) | |
| iNHL | 31 (14.8) |
| FL | 24 (11.4) |
| iNHL (not otherwise specified) | 1 (0.5) |
| MZL | 2 (1.0) |
| WM/LPL | 4 (1.9) |
| CLL/SLL (RR) | 55 (26.2) |
| CLL | 50 (23.8) |
| SLL | 5 (2.4) |
| CLL (TN) | 18 (8.6) |
| T-cell NHL | 35 (16.7) |
| Cutaneous TCL | 19 (9.0) |
| Peripheral TCL | 16 (7.6) |
| Other diagnoses | 71 (33.8) |
| aNHL | 26 (12.4) |
| MCL | 10 (4.8) |
| AML | 6 (2.9) |
| MM | 3 (1.4) |
| HL | 3 (1.4) |
| Myelofibrosis | 5 (2.4) |
| MDS | 6 (2.9) |
| T-cell ALL | 5 (2.4) |
| B-cell ALL | 4 (1.9) |
| LGL | 3 (1.4) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; aNHL, aggressive non-Hodgkin lymphoma; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; LGL, large granular lymphocytic leukemia; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MZL, marginal zone lymphoma; WM, Waldenström macroglobulinemia.
Bold entries in patient column denote disease subtypes represented in the patient population.
The median duration of duvelisib treatment was 16 weeks (range, 0.3-166.6) across all doses. Twenty of the 55 patients with RR CLL/small lymphoblastic lymphoma (SLL) (36.4%) and 12 of the 18 patients with TN CLL (66.7%) stayed on duvelisib for >1 year (or at least 12 cycles of 28 days each). Table 2 includes median exposure in the major cohorts at 2, 6, and 12 months.
Exposure of major cohorts to duvelisib
| Cohort diagnosis . | Total (N = 210), n (%) . | Subjects who began 2/6/12 cycles (28 d) (%) . | Median cycles begun . |
|---|---|---|---|
| iNHL | 31 (14.8) | 27/15/8 (87.1/48.4/25.8) | 5 |
| CLL/SLL (RR) | 55 (26.2) | 54/34/20 (98.2/61.8/36.4) | 6 |
| CLL (TN) | 18 (8.6) | 17/15/12 (94.4/83.3/66.7) | 16 |
| T-cell NHL | 35 (16.7) | 26/11/3 (74.3/31.4/8.6) | 4 |
| Cohort diagnosis . | Total (N = 210), n (%) . | Subjects who began 2/6/12 cycles (28 d) (%) . | Median cycles begun . |
|---|---|---|---|
| iNHL | 31 (14.8) | 27/15/8 (87.1/48.4/25.8) | 5 |
| CLL/SLL (RR) | 55 (26.2) | 54/34/20 (98.2/61.8/36.4) | 6 |
| CLL (TN) | 18 (8.6) | 17/15/12 (94.4/83.3/66.7) | 16 |
| T-cell NHL | 35 (16.7) | 26/11/3 (74.3/31.4/8.6) | 4 |
MTD
The DEP enrolled 31 patients who received duvelisib at 1 of 8 dose levels (range, 8 to 100 mg twice daily) (Figure 2). Four patients experienced a DLT: 1 grade 4 neutropenia at 15 mg twice daily; 1 grade 3 cellulitis at 75 mg twice daily; 1 patient had 2 grade 3 DLTs (increases in alanine transaminase [ALT] and aspartate transaminase [AST]); and 1 patient had a rash at 100 mg twice daily. Based on these findings, the MTD was determined to be 75 mg twice daily (Table 3).
Study design. The first row of boxes shows the dose escalation cohort (n = 31) and the 4 dose-limiting toxicities of duvelisib (1 at 15 mg BID, 1 at 75 mg BID, and 2 at 100 mg BID). The MTD was found to be 75 mg BID. The lower 2 boxes represent the expansion cohorts (1 at 25 mg BID and 1 at 75 mg BID).
Study design. The first row of boxes shows the dose escalation cohort (n = 31) and the 4 dose-limiting toxicities of duvelisib (1 at 15 mg BID, 1 at 75 mg BID, and 2 at 100 mg BID). The MTD was found to be 75 mg BID. The lower 2 boxes represent the expansion cohorts (1 at 25 mg BID and 1 at 75 mg BID).
Patients in the DEP and corresponding DLTs (dose escalation set)
| Duvelisib dose . | Patient no. . | Disease subtype . | DLT . |
|---|---|---|---|
| 8 mg BID | 1 | RR CLL/SLL | — |
| 15 mg BID | 2 | RR CLL/SLL | Grade 4 neutropenia |
| 3 | iNHL | — | |
| 4 | MM | — | |
| 5 | aNHL | — | |
| 6 | MM | — | |
| 7 | RR CLL/SLL | — | |
| 25 mg BID | 8 | iNHL | — |
| 9 | aNHL | — | |
| 10 | RR CLL/SLL | — | |
| 11 | MCL | — | |
| 12 | TCL | — | |
| 13 | RR CLL/SLL | — | |
| 14 | iNHL | — | |
| 35 mg BID | 15 | HL | — |
| 16 | aNHL | — | |
| 17 | MM | — | |
| 50 mg BID | 18 | iNHL | — |
| 19 | TCL | — | |
| 20 | HL | — | |
| 60 mg BID | 21 | TCL | — |
| 22 | TCL | — | |
| 23 | TCL | — | |
| 75 mg BID | 24 | aNHL | — |
| 25 | TCL | — | |
| 26 | TCL | Grade 3 cellulitis | |
| 27 | HL | — | |
| 28 | aNHL | — | |
| 29 | aNHL | — | |
| 100 mg BID | 30 | aNHL | Grade 3 ALT elevation |
| Grade 3 AST elevation | |||
| 31 | TCL | Grade 3 rash (maculopapular) |
| Duvelisib dose . | Patient no. . | Disease subtype . | DLT . |
|---|---|---|---|
| 8 mg BID | 1 | RR CLL/SLL | — |
| 15 mg BID | 2 | RR CLL/SLL | Grade 4 neutropenia |
| 3 | iNHL | — | |
| 4 | MM | — | |
| 5 | aNHL | — | |
| 6 | MM | — | |
| 7 | RR CLL/SLL | — | |
| 25 mg BID | 8 | iNHL | — |
| 9 | aNHL | — | |
| 10 | RR CLL/SLL | — | |
| 11 | MCL | — | |
| 12 | TCL | — | |
| 13 | RR CLL/SLL | — | |
| 14 | iNHL | — | |
| 35 mg BID | 15 | HL | — |
| 16 | aNHL | — | |
| 17 | MM | — | |
| 50 mg BID | 18 | iNHL | — |
| 19 | TCL | — | |
| 20 | HL | — | |
| 60 mg BID | 21 | TCL | — |
| 22 | TCL | — | |
| 23 | TCL | — | |
| 75 mg BID | 24 | aNHL | — |
| 25 | TCL | — | |
| 26 | TCL | Grade 3 cellulitis | |
| 27 | HL | — | |
| 28 | aNHL | — | |
| 29 | aNHL | — | |
| 100 mg BID | 30 | aNHL | Grade 3 ALT elevation |
| Grade 3 AST elevation | |||
| 31 | TCL | Grade 3 rash (maculopapular) |
AEs were coded using Medical Dictionary for Regulatory Activities, version 16.1, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
MM = multiple myeloma.
*Patient number represents the order in which patients were enrolled in the study.
PK results
PK analysis included 207 patients from the DEP and the expansion phase who received duvelisib at doses ranging from 8 to 100 mg twice daily. After a single dose (C1D1), duvelisib was rapidly absorbed, reaching peak concentration 1 to 2 hours later. After multiple doses, maximum plasma concentrations ranged from 471 to 3294 ng/mL, and systemic exposure (based on AUC0-12) ranged from 2001 to 19059 ng/h per milliliter. A threefold change in the dose (25 to 75 mg) resulted in a 2.2- and 2.4-fold change in Cmax and AUC, respectively. Duvelisib clearance was 3.6 to 11.2 L/h; volume of distribution was 26 to 102 L. Duvelisib half-life after a single dose was 5.2 to 10.9 hours (Figure 1; supplemental Table 1).
The rate of accumulation ranged from 1.5 to 2.9 (except for the 60 mg twice daily group, whose rate was 0.9) and the 100 mg twice daily group (whose rate was not calculated), suggesting a 1.5- to approximately threefold increase in exposure to duvelisib with repeat administration (Figure 1; supplemental Table 2).
PD results
A single dose of duvelisib 25 or 75 mg induced rapid inhibition of p-AKT (S473), a key mediator of PI3K signaling,13 in CLL tumor cells, which remained suppressed compared with baseline in the majority of samples at 24 hours (Figure 3). In addition, near-complete inhibition of CLL cell proliferation (assessed by Ki-67 expression at C2D1) was observed in nearly all patients with CLL. Neither Ki-67 nor p-AKT inhibition was dose-dependent, and both were maximal at 25 mg.
Reductions in key pharmacodynamic markers of inhibition and proliferation in CLL cells from patients with RR CLL/SLL and TN CLL. Phosphorylation of AKT at S473 was measured by flow cytometry in CD19+/CD5+ CLL cells obtained from whole blood collected at baseline (0 h) and at 1 h and 24 h postdose on C1D1. Ki-67, a marker of cell proliferation, was measured by flow cytometry on CD19+/CD5+ CLL cells obtained from whole blood collected predose (0 h) on C1D1 and C2D1). All data shown as percent positive.
Reductions in key pharmacodynamic markers of inhibition and proliferation in CLL cells from patients with RR CLL/SLL and TN CLL. Phosphorylation of AKT at S473 was measured by flow cytometry in CD19+/CD5+ CLL cells obtained from whole blood collected at baseline (0 h) and at 1 h and 24 h postdose on C1D1. Ki-67, a marker of cell proliferation, was measured by flow cytometry on CD19+/CD5+ CLL cells obtained from whole blood collected predose (0 h) on C1D1 and C2D1). All data shown as percent positive.
Additionally, serum cytokine and chemokine levels associated with the tumor microenvironment were analyzed in 54 patients with CLL and 24 patients with iNHL. Nine of the 72 serum analytes tested decreased significantly from baseline to C1D8 in CLL (P < .0007) and exhibited a significant ≥30% median change in iNHL (P < .05): CCL1, CCL4, CCL17, CCL22, CXCL10, CXCL13, interleukin-10, matrix metalloproteinase-9, and tumor necrosis factor-α. Eight of the 9 analytes (except interleukin-10 in iNHL) were elevated in patient serum at baseline compared with healthy donors (n = 33).
Safety results
Among the entire study population (DEP and expansion phase, n = 210), the most frequent (>15% patients overall or >5% ≥grade 3) all-causality, treatment-emergent AEs are presented in Table 4. Most events were grade 1 or 2, with a few exceptions. Although the study was not statistically designed to detect significant differences between dose levels, there did not appear to be a notable difference in the safety profile of duvelisib by dose. The 2 largest dose cohorts (25 mg twice daily and 75 mg twice daily) showed similar rates of severe (grade ≥3) AEs (80% vs 87%), AEs investigator-assessed as related to duvelisib (85% vs 91%), and AEs leading to treatment discontinuation (27% vs 36%).
Incidence of AEs (>15% of patients overall or >5% ≥grade 3)
| AE category, SOC, PT . | All patients (all duvelisib doses) (N = 210), n (%) . | ||
|---|---|---|---|
| Grade | Any | 3 | 4 |
| Hematologic | |||
| Neutropenia | 81 (38.6) | 19 (9.0) | 23 (11.0) |
| Anemia | 52 (24.8) | 29 (13.8) | 1 (0.5) |
| Thrombocytopenia/decreased platelets | 49 (23.3) | 9 (4.3) | 21 (10.0) |
| Investigations | |||
| ALT increased | 81 (38.6) | 33 (15.7) | 8 (3.8) |
| AST increased | 79 (37.6) | 30 (14.3) | 2 (1.0) |
| Hypokalemia | 31 (14.8) | 5 (2.4) | 6 (2.9) |
| Nonhematologic | |||
| Diarrhea | 88 (41.9) | 24 (11.4) | 0 |
| Fatigue | 85 (40.5) | 17 (8.1) | 1 (0.5) |
| Pyrexia | 74 (35.2) | 3 (1.4) | 0 |
| Nausea | 67 (31.9) | 7 (3.3) | 0 |
| Cough | 66 (31.4) | 1 (0.5) | 0 |
| Decreased appetite | 44 (21.0) | 2 (1.0) | 0 |
| Edema peripheral | 43 (20.5) | 1 (0.5) | 0 |
| Dyspnea | 41 (19.5) | 9 (4.3) | 2 (1.0) |
| Headache | 38 (18.1) | 4 (1.9) | 0 |
| Vomiting | 37 (17.6) | 4 (1.9) | 0 |
| Arthralgia | 35 (16.7) | 4 (1.9) | 0 |
| Rash maculopapular | 34 (16.2) | 11 (5.2) | 0 |
| Upper respiratory tract infection | 34 (16.2) | 1 (0.5) | 0 |
| Rash | 30 (14.3) | 1 (0.5) | 0 |
| Constipation | 32 (15.2) | 1 (0.5) | 0 |
| Pneumonia | 28 (13.3) | 19 (9.0) | 1 (0.5) |
| AE category, SOC, PT . | All patients (all duvelisib doses) (N = 210), n (%) . | ||
|---|---|---|---|
| Grade | Any | 3 | 4 |
| Hematologic | |||
| Neutropenia | 81 (38.6) | 19 (9.0) | 23 (11.0) |
| Anemia | 52 (24.8) | 29 (13.8) | 1 (0.5) |
| Thrombocytopenia/decreased platelets | 49 (23.3) | 9 (4.3) | 21 (10.0) |
| Investigations | |||
| ALT increased | 81 (38.6) | 33 (15.7) | 8 (3.8) |
| AST increased | 79 (37.6) | 30 (14.3) | 2 (1.0) |
| Hypokalemia | 31 (14.8) | 5 (2.4) | 6 (2.9) |
| Nonhematologic | |||
| Diarrhea | 88 (41.9) | 24 (11.4) | 0 |
| Fatigue | 85 (40.5) | 17 (8.1) | 1 (0.5) |
| Pyrexia | 74 (35.2) | 3 (1.4) | 0 |
| Nausea | 67 (31.9) | 7 (3.3) | 0 |
| Cough | 66 (31.4) | 1 (0.5) | 0 |
| Decreased appetite | 44 (21.0) | 2 (1.0) | 0 |
| Edema peripheral | 43 (20.5) | 1 (0.5) | 0 |
| Dyspnea | 41 (19.5) | 9 (4.3) | 2 (1.0) |
| Headache | 38 (18.1) | 4 (1.9) | 0 |
| Vomiting | 37 (17.6) | 4 (1.9) | 0 |
| Arthralgia | 35 (16.7) | 4 (1.9) | 0 |
| Rash maculopapular | 34 (16.2) | 11 (5.2) | 0 |
| Upper respiratory tract infection | 34 (16.2) | 1 (0.5) | 0 |
| Rash | 30 (14.3) | 1 (0.5) | 0 |
| Constipation | 32 (15.2) | 1 (0.5) | 0 |
| Pneumonia | 28 (13.3) | 19 (9.0) | 1 (0.5) |
AEs were coded using Medical Dictionary for Regulatory Activities, version 16.1, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
PT, preferred term; SOC, system organ class.
As expected for this patient population, hematologic AEs (neutropenia, anemia, thrombocytopenia) were relatively common. Neutropenia was the most frequent, occurring in 39% of patients, with 32% of patients experiencing ≥grade 3 events. However, these events infrequently necessitated treatment modifications, were rarely serious, and resulted in treatment discontinuation in only 2 patients.
Transaminase (ALT/AST) elevations (39/38%) and diarrhea (42%) were the most frequent nonhematologic AEs overall and the most common nonhematologic events ≥grade 3. Although the AE profile in individual disease cohorts was generally consistent with the whole study population, the incidences of diarrhea in TN CLL patients (78%) and transaminase elevations in TCL (57%) and iNHL (58%) may reflect cohort-specific differences. AST and ALT increases were observed early in treatment, with a median time to onset of first occurrence of 1.2 months. For severe transaminase elevations, median duration was approximately 2 weeks. Most patients with transaminase elevations were managed with dose modifications, and few patients (n = 14; 7%) discontinued treatment because of these events. For diarrhea, the median time to first occurrence (any grade) was 2.2 months, whereas the median time to severe diarrhea (≥grade 3) was 4.9 months. Severe events of colitis and pneumonitis were reported in 12 (6%) and 9 (4%) patients, respectively.
Not surprisingly given the patient population, infections of all grades were common, reported in 128 (61%) patients. The most frequent infection AE was upper respiratory tract infection, in 34 (16.2%) patients. Pneumonia (28 patients, 13% overall) was the only infection AE to occur at ≥grade 3 severity in >5% of patients (severe events in n = 20 or 10% of patients). Additionally, Pneumocystis jirovecii pneumonia was reported in 3 patients (1%, none of whom was on prophylaxis before infection) and cytomegalovirus infection in 2 patients (1%).
All 11 patients (5%) who experienced fatal AEs other than disease progression had RR disease, including RR CLL (5 patients), iNHL, DLBCL, and TCL (2 patients each). There were no fatal treatment-emergent AEs in TN CLL patients. Eight patients with fatal infections had received 3 to 9 prior therapies, many of which were chemoimmunotherapy regimens, and half had already discontinued duvelisib because of progressive disease before the onset of the AE. Infectious treatment-emergent AEs included 2 cases of P jirovecii pneumonia (before the enactment of the protocol amendment requiring Pneumocystis prophylaxis at all investigative sites). The remaining infectious fatal events, each in a single patient, were Escherichia sepsis, various pneumonias (fungal, herpes viral, Pseudomonas aeruginosa, respiratory syncytial viral), and pseudomonal sepsis. Three patients died of noninfectious AEs: 1 of metabolic acidosis (assessed as treatment related) and 2 of respiratory failure.
Efficacy results
Response was reported per investigator assessment using disease-specific response criteria. The ORR in patients with RR CLL (n = 55) and TN CLL (n = 18) was 56% and 83%, respectively, and included 1 CR in an RR patient. In patients with iNHL (n = 31), the ORR was 58%, which included 6 CRs. Among TCL patients, responses (all PRs) were observed in 32% of patients with cutaneous TCL (n = 19) and 50% (including 3 CRs) of patients with peripheral TCL (n = 16). As additional evidence of duvelisib efficacy in hematologic malignancies, responses were observed in other small disease cohorts, including CRs in patients with myelodysplastic syndrome, mantle cell lymphoma, and aggressive NHL (Table 5).
Summary of best overall response, ORR, and progression-free survival by disease subtype
| . | . | Duvelisib dose (mg, BID), n (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Total . | 8 . | 15 . | 25 . | 35 . | 50 . | 60 . | 75 . | 100 . |
| RR CLL/SLL, n | 55 | 1 | 2 | 28 | 0 | 0 | 0 | 24 | 0 |
| ORR, n (%) | 31 (56) | 1 (100) | 1 (50) | 16 (57) | — | — | — | 13 (54) | — |
| CR | 1 (2) | 0 | 0 | 1 (4) | — | — | — | 0 | — |
| PR | 30 (55) | 1 (100) | 1 (50) | 15 (54) | — | — | — | 13 (54) | — |
| Median months of PFS (95% CI) | 15.7 (5.4-30.2) | ||||||||
| TN CLL, n | 18 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 |
| ORR, n (%) | 15 (83) | — | — | 15 (83) | — | — | — | — | — |
| PR | 15 (83) | — | — | 15 (83) | — | — | — | — | — |
| Median months of PFS (95% CI) | NE (12.7-NE) | ||||||||
| iNHL, n | 31 | 0 | 1 | 14 | 0 | 1 | 0 | 15 | 0 |
| ORR, n (%) | 18 (58) | — | 1 (100) | 9 (64) | — | 1 (100) | — | 7 (47) | — |
| CR | 6 (19) | — | 1 (100) | 4 (29) | — | 0 | — | 1 (7) | — |
| PR | 11 (36) | — | — | 4 (29) | — | 1 (100) | — | 6 (40) | — |
| MR | 1 (3) | — | — | 1 (7) | — | 0 | — | 0 | — |
| Median months of PFS (95% CI) | 14.7 (5.4-30.2) | ||||||||
| aNHL, n* | 26 | 0 | 1 | 1 | 1 | 0 | 0 | 22 | 1 |
| ORR, n (%) | 5 (19) | — | 0 | 0 | 0 | — | — | 5 (23) | 0 |
| CR | 2 (8) | — | 0 | 0 | 0 | — | — | 2 (9) | 0 |
| PR | 3 (12) | — | 0 | 0 | 0 | — | — | 3 (14) | 0 |
| MCL, n | 10 | 0 | 0 | 4 | 0 | 0 | 0 | 6 | 0 |
| ORR n (%) | 5 (50) | — | — | 3 (75) | — | — | — | 2 (33) | — |
| CR | 1 (10) | — | — | 1 (25) | — | — | — | 0 | — |
| PR | 4 (40) | — | — | 2 (50) | — | — | — | 2 (33) | — |
| HL, n | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| ORR, n (%) | 1 (33) | — | — | — | 0 | 1 (100) | — | 0 | — |
| CR | 1 (33) | — | — | — | 0 | 1 (100) | — | 0 | — |
| Cutaneous TCL, n | 19 | 0 | 0 | 1 | 0 | 1 | 2 | 14 | 1 |
| ORR, n (%) | 6 (32) | — | — | 0 | — | 0 | 1 (50) | 5 (36) | 0 |
| PR | 6 (32) | — | — | 0 | — | 0 | 1 (50) | 5 (36) | 0 |
| Median months of PFS (95% CI) | 4.5 (1.0-5.6) | ||||||||
| Peripheral TCL, n | 16 | 0 | 0 | 0 | 0 | 0 | 2 | 13 | 1 |
| ORR, n (%) | 8 (50) | — | — | — | — | — | 1 (50) | 7 (54) | 0 |
| CR | 3 (19) | — | — | — | — | — | 1 (50) | 2 (15) | 0 |
| PR | 5 (31) | — | — | — | — | — | 1 (50) | 2 (15) | 0 |
| Median months of PFS (95% CI) | 4.4 (0.7-NE) | ||||||||
| AML, n | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 |
| ORR, n (%) | 0 | — | — | — | — | — | — | 0 | — |
| B-cell ALL, n | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| ORR, n (%) | 0 | — | — | — | — | — | — | 0 | — |
| LGL, n | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| ORR, n (%) | 1 (33) | — | — | — | — | — | — | 1 (33) | — |
| PR | 1 (33) | — | — | — | — | — | — | 1 (33) | — |
| MM, n | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| ORR n (%) | 0 | — | 0 | — | 0 | — | — | — | - |
| MDS, n | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 |
| ORR, n (%) | 1 (17) | — | — | — | — | — | — | 1 (17) | — |
| CR | 1 (17) | — | — | — | — | — | — | 1 (17) | — |
| Myelofibrosis, n | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| ORR, n (%) | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| Clinical Improvement | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| T-cell ALL, n | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| ORR, n (%) | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| PR | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| . | . | Duvelisib dose (mg, BID), n (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Total . | 8 . | 15 . | 25 . | 35 . | 50 . | 60 . | 75 . | 100 . |
| RR CLL/SLL, n | 55 | 1 | 2 | 28 | 0 | 0 | 0 | 24 | 0 |
| ORR, n (%) | 31 (56) | 1 (100) | 1 (50) | 16 (57) | — | — | — | 13 (54) | — |
| CR | 1 (2) | 0 | 0 | 1 (4) | — | — | — | 0 | — |
| PR | 30 (55) | 1 (100) | 1 (50) | 15 (54) | — | — | — | 13 (54) | — |
| Median months of PFS (95% CI) | 15.7 (5.4-30.2) | ||||||||
| TN CLL, n | 18 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 |
| ORR, n (%) | 15 (83) | — | — | 15 (83) | — | — | — | — | — |
| PR | 15 (83) | — | — | 15 (83) | — | — | — | — | — |
| Median months of PFS (95% CI) | NE (12.7-NE) | ||||||||
| iNHL, n | 31 | 0 | 1 | 14 | 0 | 1 | 0 | 15 | 0 |
| ORR, n (%) | 18 (58) | — | 1 (100) | 9 (64) | — | 1 (100) | — | 7 (47) | — |
| CR | 6 (19) | — | 1 (100) | 4 (29) | — | 0 | — | 1 (7) | — |
| PR | 11 (36) | — | — | 4 (29) | — | 1 (100) | — | 6 (40) | — |
| MR | 1 (3) | — | — | 1 (7) | — | 0 | — | 0 | — |
| Median months of PFS (95% CI) | 14.7 (5.4-30.2) | ||||||||
| aNHL, n* | 26 | 0 | 1 | 1 | 1 | 0 | 0 | 22 | 1 |
| ORR, n (%) | 5 (19) | — | 0 | 0 | 0 | — | — | 5 (23) | 0 |
| CR | 2 (8) | — | 0 | 0 | 0 | — | — | 2 (9) | 0 |
| PR | 3 (12) | — | 0 | 0 | 0 | — | — | 3 (14) | 0 |
| MCL, n | 10 | 0 | 0 | 4 | 0 | 0 | 0 | 6 | 0 |
| ORR n (%) | 5 (50) | — | — | 3 (75) | — | — | — | 2 (33) | — |
| CR | 1 (10) | — | — | 1 (25) | — | — | — | 0 | — |
| PR | 4 (40) | — | — | 2 (50) | — | — | — | 2 (33) | — |
| HL, n | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| ORR, n (%) | 1 (33) | — | — | — | 0 | 1 (100) | — | 0 | — |
| CR | 1 (33) | — | — | — | 0 | 1 (100) | — | 0 | — |
| Cutaneous TCL, n | 19 | 0 | 0 | 1 | 0 | 1 | 2 | 14 | 1 |
| ORR, n (%) | 6 (32) | — | — | 0 | — | 0 | 1 (50) | 5 (36) | 0 |
| PR | 6 (32) | — | — | 0 | — | 0 | 1 (50) | 5 (36) | 0 |
| Median months of PFS (95% CI) | 4.5 (1.0-5.6) | ||||||||
| Peripheral TCL, n | 16 | 0 | 0 | 0 | 0 | 0 | 2 | 13 | 1 |
| ORR, n (%) | 8 (50) | — | — | — | — | — | 1 (50) | 7 (54) | 0 |
| CR | 3 (19) | — | — | — | — | — | 1 (50) | 2 (15) | 0 |
| PR | 5 (31) | — | — | — | — | — | 1 (50) | 2 (15) | 0 |
| Median months of PFS (95% CI) | 4.4 (0.7-NE) | ||||||||
| AML, n | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 |
| ORR, n (%) | 0 | — | — | — | — | — | — | 0 | — |
| B-cell ALL, n | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| ORR, n (%) | 0 | — | — | — | — | — | — | 0 | — |
| LGL, n | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| ORR, n (%) | 1 (33) | — | — | — | — | — | — | 1 (33) | — |
| PR | 1 (33) | — | — | — | — | — | — | 1 (33) | — |
| MM, n | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| ORR n (%) | 0 | — | 0 | — | 0 | — | — | — | - |
| MDS, n | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 |
| ORR, n (%) | 1 (17) | — | — | — | — | — | — | 1 (17) | — |
| CR | 1 (17) | — | — | — | — | — | — | 1 (17) | — |
| Myelofibrosis, n | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| ORR, n (%) | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| Clinical Improvement | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| T-cell ALL, n | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| ORR, n (%) | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
| PR | 1 (20) | — | — | — | — | — | — | 1 (20) | — |
Percentages are based on the number of patients in each dose group.
DLBCL, diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = Hodgkin lymphoma; MR = minor response; NE, not estimable; PFS = progression-free survival; PR = partial response.
aNHL includes the subtypes of Richter transformation (n = 9), DLBCL (n = 14), transformed FL (n = 2), and DLBCL transformation (n = 1).
Discussion
In this phase 1, open-label, dose-escalation study, the PK, PD, safety, and efficacy of duvelisib were evaluated in patients representing a broad range of advanced hematologic malignancies. Based upon the occurrence of 2 DLTs at 100 mg twice daily, the MTD of duvelisib was determined to be 75 mg twice daily. Duvelisib demonstrated in vivo pharmacologic activity through rapid and sustained reductions in p-AKT, a downstream marker of PI3K signaling and reductions in Ki-67 expression, early in treatment (C2D1). Maximal p-AKT and Ki-67 effects were achieved at 25 mg twice daily, with higher duvelisib doses and/or plasma concentrations not providing further suppression. These findings, as well as the clinically meaningful activity at 25mg twice daily, informed the decision to open expansion phase cohorts at this dose and to ultimately advance it for further investigation in phase 2 and 3 studies. Duvelisib is rapidly absorbed after multiple-dose administration (time to Cmax, 1-3 hours) and shows a nearly dose-dependent PK profile whereby a threefold increase in dose resulted in an approximately twofold increase in Cmax and AUC. Duvelisib is widely distributed to peripheral tissues (as indicated by a large volume of distribution) and is eliminated with a half-life of 2.7 to 7.5 hours. Most of the compound is hepatically transformed into its inactive major metabolite, IPI-656, before enteric elimination.
Recent preclinical and early clinical data on a PI3K-γ–specific inhibitor suggest an acceptable safety profile in patients with advanced malignancies.14,15 Within the context of dual PI3K-δ,γ inhibition, the AE profile of duvelisib was manageable, with most treatment-emergent AEs presenting as low-grade events (grade 1-2). Many commonly observed AEs, such as infections and cytopenias, are expected in a heavily pretreated population with advanced hematologic malignancies. There were 8 deaths from infectious events, all in heavily pretreated patients, half of which occurred in the setting of disease progression. Immune system dysfunction inherent to advanced hematologic malignancies and persisting from prior therapy confounds the relative contribution of duvelisib treatment to infectious complications.16 However, the preliminary safety data across this diverse patient population informed risk mitigation guidance, including dosing modifications and mandatory Pneumocystis prophylaxis, for the subsequent phase 2 (iNHL)17 and phase 3 (CLL/SLL) studies with duvelisib from which a more robust clinical risk-benefit assessment may be derived.
Consistent with observations in studies of PI3K inhibitor therapy, transaminase (ALT/AST) elevations and diarrhea were frequent. These events, even when severe, were often manageable with dose modifications and rarely resulted in treatment discontinuation. Transaminase elevations were observed somewhat early in treatment, within the first 2 months, and did not appear to be dose dependent. Severe diarrhea events tended to occur later, approximately 5 to 6 months into treatment, and also did not appear to be dose dependent. Diarrhea and colitis represent a clinicopathologic spectrum of toxicity associated with PI3K-δ inhibitor therapy.2 The absence of universal colonoscopy evaluations for all severe diarrhea events may confound estimation of colitis incidence. With regard to safety across disease populations, diarrhea incidence was similar across relapsed disease groups but higher in TN CLL, and transaminase elevations were less frequent in RR CLL than in iNHL or TCL (data not shown), suggesting toxicity modulation by disease-specific factors, or differences in prior treatments or immune-cell populations. These findings are consistent with results from studies of other PI3K-δ inhibitors, such as idelalisib.18,19
The complex interplay between tumor cells and the tumor microenvironment and the clinical utility of its disruption have been the focus of investigation. In this study, significant on-treatment reductions of key serum cytokines and chemokines expressed by malignant B cells12 and tumor-supporting myeloid and T cells were observed. These data suggest potential pharmacodynamic effects of duvelisib on the tumor microenvironment and warrant additional characterization within the context of specific lymphoid malignancies in future studies.4,20-25
The clinical activity with duvelisib treatment across the various disease populations enrolled in this phase 1 study was consistent with the current understanding of the contributions of PI3K-δ and PI3K-γ to the pathogenesis and maintenance of hematologic malignancies. Responses were noted across the dose range evaluated and in nearly all groups examined. Response rates were particularly noteworthy in patients with RR iNHL and CLL, in whom respective ORR of 58% and 56% represent meaningful clinical activity. In RR TCL, ORR of 50% in patients with peripheral TCL and 32% in those with cutaneous TCL are also compelling given the modest response rates of approved single agents, which range from 25% to 29%.26-28 The underlying mechanism for this observed sensitivity is unknown. In addition to the importance of PI3K-δ/γ in T-cell functions,8,22,29-31 an additional mechanism may be a tumor microenvironment disruption induced by PI3K-δ/PI3K-γ inhibition; in some peripheral TCL subtypes, particularly angioimmunoblastic TCL, molecular profiling has identified microenvironmental signatures further associated with poor outcome.32 Given the activity observed in this study, further characterization of duvelisib’s clinical and biologic effects in TCL is warranted. Accordingly, a phase 2 study in TCL is under development.
Despite progress in extending survival in patients with advanced hematologic malignancies, RR advanced-stage B-cell lymphoma and TCL remain largely incurable, requiring life-long management of both the disease and its associated morbidity. The simultaneous targeting of cell-autonomous survival pathways and disruption of the complex interplay between tumor cells and their microenvironment through combined PI3K-δ and PI3K-γ inhibition may represent another therapeutic alternative. In extensively pretreated patients with RR leukemia or lymphoma and previously untreated CLL, duvelisib monotherapy demonstrated clinically meaningful activity and an acceptable safety profile. Based on the findings from the DEP and the expansion phase, 25 mg twice daily duvelisib was selected for future evaluation in phase 2 and 3 studies. This decision was supported in part by the PD findings, which showed that duvelisib plasma exposure at doses >25 mg twice daily did not result in increased suppression of p-AKT or Ki-67, 2 downstream markers of PI3K-δ inhibition. In addition, response rates were not higher with doses >25 mg twice daily.
In summary, findings from this phase 1 study, the first clinical evaluation of duvelisib, an oral dual inhibitor of PI3K-δ and PI3K-γ, support continued development as a potential contributor to the lymphoma and leukemia treatment paradigm. To further assess duvelisib 25 mg twice daily monotherapy, 2 studies are in progress: Duvelisib in Subjects With Refractory Indolent Non-Hodgkin Lymphoma (NCT01882803), a phase 2 study in patients with relapsed iNHL, and Duvelisib Versus Ofatumumab in Patients With Relapsed or Refractory CLL/SLL (NCT02004522), a phase 3 study in patients with RR CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study investigators, coordinators, nurses, and patients and their families for their contributions. Steven Mousterakis and Justin McLaughlin, formerly of Infinity Pharmaceuticals, Inc., and Paul Guttry of Acumen Medical Communications, provided editorial and graphics support.
This work was supported by Infinity Pharmaceuticals, Inc., Verastem, and a grant from the National Institutes of Health, National Cancer Institute Cancer Center (P30 CA008748).
Authorship
Contribution: I.W.F. provided primary authorship and data interpretation; I.W.F., B.K., and P.K. designed the trial; I.W.F., S.O., B.K., M.P., Y.O., F.F.F., P.P., J.J., J.A.B., N.J., and S.H. performed the research and contributed to/edited the manuscript; V.M.K. and J.S. analyzed data and wrote and/or edited the manuscript; K.A. provided statistical outputs; and M.D. performed pharmacodynamic analysis and data interpretation.
Conflict-of-interest disclosure: I.W.F. has received funding/grant support from Acerta Pharma, Agios Pharmaceuticals, BeiGene, Celgene, Constellation Pharmaceuticals, Curis Inc, Forma Therapeutics, Forty Seven Inc., Genentech, Gilead Sciences, Incyte Corporation, Infinity Pharmaecuticals, Janssen Pharmaceutical, Kite Pharma, Merck, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics, Takeda Pharmaceuticals, TG Therapeutics, Inc., Trillium Therapeutics, Inc., and Verastem Inc. Y.O. received honoraria from Bristol-Myers Squibb and Takeda, and research funding from Infinity Pharmaceuticals, Inc., Rhizen Pharmaceuticals S.A., and Curis Inc. S.H. has received research funding/grant support from Celgene Corporation, Millennium Pharmaceuticals, Inc., Seattle Genetics, Spectrum Pharmaceuticals, Inc., and Infinity Pharmaceuticals, Inc., and consulting fees/honoraria from Celgene Corporation, Millennium Pharmaceuticals, Inc., Seattle Genetics, and Spectrum Pharmaceuticals, Inc. F.F.F. has received consulting fees and funding for a clinical study from Infinity Pharmaceuticals, Inc. K.A., M.D., J.S., and P.K. are former employees of Infinity Pharmaceuticals, Inc. B.K. has received consulting fees from Infinity Pharmaceuticals, Inc., Gilead Sciences, Inc., Pharmacyclics, Roche, Millennium Pharmaceuticals, Inc., Seattle Genetics, and CTI BioPharma. V.M.K. is a former employee of Infinity Pharmaceuticals, Inc., and currently a consultant for Verastem, Inc. The remaining authors declare no competing financial interests.
The current affiliation for S.O. is University of California Irvine Health Chao Family Comprehensive Cancer Center, Orange, CA.
The current affiliation for B.K. is Washington University School of Medicine, St. Louis, MO.
The current affiliation for P.P. is Sidney Kimmel Cancer Center at Thomas Jefferson University, Philadelphia, PA.
The current affiliation for J.J. is Celgene Pharmaceuticals, Summit, NJ.
Correspondence: Ian W. Flinn, Sarah Cannon Research Institute, 250 25th Ave North, Nashville, TN 37203; e-mail: iflinn@tnonc.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal