In this issue of Blood, Bachy et al1 have confirmed the prognostic validity of the follicular lymphoma international prognostic index (FLIPI)2 in the immunochemotherapy era and have developed a simpler prognostic index with similar discriminatory power.
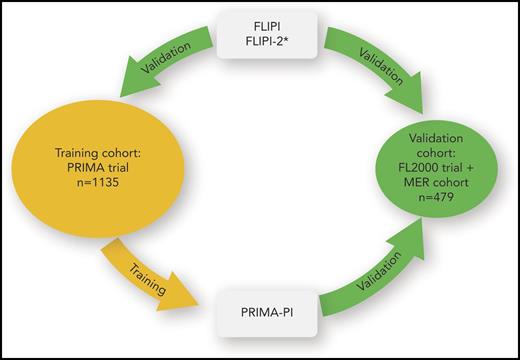
Training and validation of prognostic models in advanced stage follicular lymphoma. The patient cohort of the Primary Rituximab and Maintenance (PRIMA) trial was used to validate and compare the prognostic capacity of FLIPI and FLIPI-2. Because the PRIMA-PI was developed on the PRIMA cohort, its prognostic capacity cannot be compared with the other prognostic models on the PRIMA cohort because of overfitting. The combined FL2000 and Molecular Epidemiology Resource (MER) cohort was used to validate the prognostic capacity of the PRIMA-PI and to compare it with FLIPI. *The FLIPI-2 could not be assessed for the FL2000 + MER cohort.
Training and validation of prognostic models in advanced stage follicular lymphoma. The patient cohort of the Primary Rituximab and Maintenance (PRIMA) trial was used to validate and compare the prognostic capacity of FLIPI and FLIPI-2. Because the PRIMA-PI was developed on the PRIMA cohort, its prognostic capacity cannot be compared with the other prognostic models on the PRIMA cohort because of overfitting. The combined FL2000 and Molecular Epidemiology Resource (MER) cohort was used to validate the prognostic capacity of the PRIMA-PI and to compare it with FLIPI. *The FLIPI-2 could not be assessed for the FL2000 + MER cohort.
Knowledge of prognostic factors (ie, patient or disease characteristics partly explaining the variability of clinical outcome) is helpful in patient management and medical research in the following ways: to estimate the clinical course expected according to a patient’s individual risk profile; to compare the prognostic profile of patient cohorts across different studies; and to adjust observed effects for potential confounders in nonrandomized studies as well as in randomized trials. Ultimately, the major goals of prognostic research are to project individual clinical outcome as accurately as possible and to find predictive factors of treatment outcome3 that give rise to risk-adapted treatment decisions.
In light of the improved outcome of patients with advanced stage follicular lymphoma in the immunochemotherapy era, one might question whether previously established prognostic classification tools such as FLIPI and FLIPI-24 still discriminate patients with respect to the clinical course. Bachy et al have validated the prognostic capacity of most relevant and widely used prognostic indices using the data of 2 large prospective cohorts of patients with follicular lymphoma treated first-line with immunochemotherapy (see figure). FLIPI consistently defined 3 distinguished prognostic groups in both cohorts; FLIPI-2, which could be evaluated on 1 cohort only, also discriminated 3 prognostic groups. Although FLIPI and FLIPI-2 are frequently assessed in studies and clinical practice, outcome data stratified by prognostic groups from larger patient cohorts treated according to current standards are still needed. The present study substantially adds to the current evidence and facilitates estimating progression-free survival according to FLIPI and FLIPI-2 in patients with follicular lymphoma treated first-line with immunochemotherapy.
FLIPI and FLIPI-2 both are based on 5 clinical factors and require measurement of the extent of nodal lymphoma involvement that may lead to less reproducible or missing values. Using the data of the PRIMA trial,5 Bachy et al aimed at developing an easily applicable prognostic classification system including fewer clinical variables. After choosing cutoffs for quantitative pretreatment characteristics based on their association with the primary outcome (ie, progression-free survival) and after selecting the clinical characteristics with highest prognostic effects in multivariable regression, they applied conditional inference trees to define 3 prognostic groups. Their new prognostic index, the PRIMA-PI, is remarkably simple, requiring information on pretreatment bone marrow involvement and serum β2-microglobulin only. Bachy et al then used a cohort combined from a clinical trial and registry of 2 clinical centers for independent validation that revealed a prognostic discriminatory capacity of PRIMA-PI comparable to the FLIPI.
FLIPI was developed for overall survival and established in the prerituximab era. In contrast, FLIPI-2 was designed for progression-free survival, and, in its development cohort, more than one-half of patients had been treated with rituximab-containing regimens. Notably, the PRIMA-PI represents a simplification of FLIPI-2, by using 2 of the 5 FLIPI-2 factors, although defining prognostic groups in a different way. From the point of view of statistical learning, predictive accuracy increases with higher model complexity, whereas the generalizability to future patients can be drastically reduced with high-dimensional data.6 To what extent the reduction of FLIPI-2 factors is at the expense of lower predictive capacity remains to be determined. A fair comparison of PRIMA-PI and FLIPI-2 using the PRIMA cohort is not possible: PRIMA-PI is expected to be overfitted to its training cohort, whereas the evaluation of FLIPI-2 on the PRIMA cohort represents an independent validation (see figure). Unfortunately, the information on lymph node size necessary for the assessment of FLIPI-2 was missing in the validation cohort for PRIMA-PI. Thus, the head-to-head comparison of PRIMA-PI and FLIPI-2 represents an open question.
The PRIMA-PI defines 3 prognostic groups with different clinical courses. Of note, the variability of outcome within the prognostic groups is still substantial. Therefore, current research focuses on integrating clinical and biological markers to improve predictive power.7 At present, many groups are generating high-dimensional data to describe the heterogeneous biology of follicular lymphoma. From the statistical learning perspective, data reduction is certainly needed to derive predictive models with acceptable generalizability to future patients. Whether a simplification of clinical data that are limited in number and have shown strong prognostic effects is a reasonable approach needs to be considered in future studies.
The discrimination of outcome according to prognostic groups shown by Bachy et al indicates that current treatment, despite high efficacy, still needs improvement. Per current treatment guidelines, prognostic indices such as FLIPI, FLIPI-2, and PRIMA-PI should not be used to decide on specific therapies for a patient. Future studies on prognostic factors and, more importantly, on markers predictive of treatment response might give rise to novel individualized treatment strategies in patients with follicular lymphoma.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal