Key Points
Manufacturing CART19 by transfecting autologous T cells with messenger RNA is feasible.
Targeting CD19+ B cells in cHL using nonviral RNA CART19 was well tolerated and resulted in transient responses in a pilot study.
Abstract
Chimeric antigen receptor (CAR)–modified T cells are being investigated in many settings, including classical Hodgkin lymphoma (cHL). The unique biology of cHL, characterized by scant Hodgkin and Reed-Sternberg (HRS) cells within an immunosuppressive tumor microenvironment (TME), may pose challenges for cellular therapies directly targeting antigens expressed on HRS cells. We hypothesized that eradicating CD19+ B cells within the TME and the putative circulating CD19+ HRS clonotypic cells using anti-CD19–directed CAR-modified T cells (CART19) may indirectly affect HRS cells, which do not express CD19. Here we describe our pilot trial using CART19 in patients with relapsed or refractory cHL. To limit potential toxicities, we used nonviral RNA CART19 cells, which are expected to express CAR protein for only a few days, as opposed to CART19 generated by viral vector transduction, which expand in vivo and retain CAR expression. All 5 enrolled patients underwent successful manufacturing of nonviral RNA CART19, and 4 were infused with protocol-specified cell dose. There were no severe toxicities. Responses were seen, but these were transient. To our knowledge, this is the first CART19 clinical trial to use nonviral RNA gene delivery. This trial was registered at www.clinicaltrials.gov as #NCT02277522 (adult) and #NCT02624258 (pediatric).
Introduction
Anti-CD19 chimeric antigen receptor (CAR) T cell (CART19) therapies have demonstrated encouraging results in several B-cell malignancies.1-4 Hodgkin and Reed-Sternberg (HRS) cells are of B-cell origin but lack CD19 expression. Nonetheless, some circulating CD19+ B cells are considered to be putative HRS stem cells.5 An additional rationale for using CART19 cells in classical Hodgkin lymphoma (cHL) is that cytokine production by CART19 engaging with nonmalignant CD19+ B cells within the tumor may alter the tumor microenvironment (TME) and break its immunosuppressive milieu.6
Most CAR T-cell studies use modified cells that are manufactured by transduction of autologous T cells with viral vectors. This method results in genomic integration of the transgene and permanent expression of CAR, which allows these cells to maintain CAR expression and expand in vivo.7 CAR T cells can also be manufactured by transfecting T cells with messenger RNA using electroporation.6,8 The latter approach results in transient expression of CAR, which limits the potential for adverse effects such as cytokine release syndrome, neurotoxicity, or B-cell aplasia. Here we report our open-label pilot study using nonviral RNA CART19 cells in patients with relapsed or refractory cHL.
Study design
Patients with biopsy-proven relapsed or refractory cHL for whom ≥1 salvage therapy had failed and who lacked curative options were eligible. The investigational agent was a nonviral RNA CART19 cell expressing an anti-CD19 single-chain variable fragment linked to 4-1BB and CD3-ζ signaling domains, as described in other studies.1,2 Patients underwent leukapheresis, followed by in vitro T-cell activation and expansion, as previously described.1,2,8 The expanded T cells were transfected with messenger RNA encoding the anti-CD19 CAR using electroporation at the end of culture followed by cryopreservation. Patients received lymphodepleting chemotherapy with cyclophosphamide 30 mg/kg within 4 days before the first CART19 infusion and on day 7 (Figure 1A). Patients then received a total of 6 CART19 infusions on days 0, 2, 4, 9, 11, and 14. CART19 cell dose was based on patient weight.
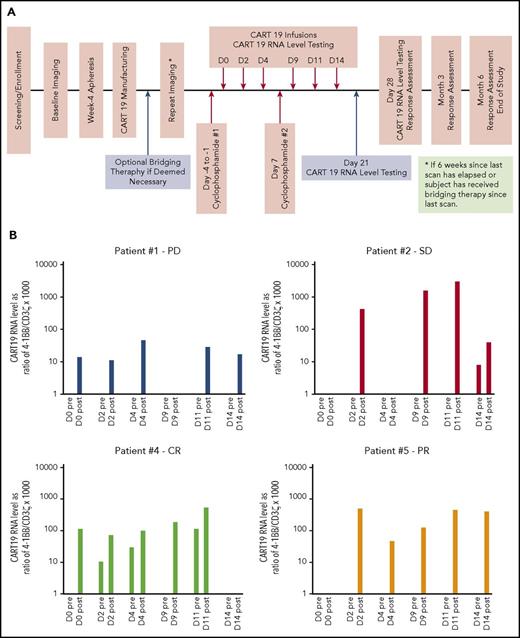
Study design and CART19 RNA persistence. (A) Study schema. (B) CART19 RNA levels in peripheral blood. These were measured on each infusion day pre- and postinfusion and expressed as normalized ratio of 4-1BB/CD3ζ gene transcript copies by reverse transcription polymerase chain reaction and multiplied by a factor of 1000. Patient 1, who experienced progressive disease (PD), had the lowest CART19 RNA levels, and no CART19 RNA was detected in preinfusion samples. This was in contrast to patient 4, who achieved complete response (CR). He had CART19 RNA detected in 3 of 5 preinfusion samples, reflecting persistence for at least 48 hours from previous infusion on those days. PR, partial response; SD, stable disease.
Study design and CART19 RNA persistence. (A) Study schema. (B) CART19 RNA levels in peripheral blood. These were measured on each infusion day pre- and postinfusion and expressed as normalized ratio of 4-1BB/CD3ζ gene transcript copies by reverse transcription polymerase chain reaction and multiplied by a factor of 1000. Patient 1, who experienced progressive disease (PD), had the lowest CART19 RNA levels, and no CART19 RNA was detected in preinfusion samples. This was in contrast to patient 4, who achieved complete response (CR). He had CART19 RNA detected in 3 of 5 preinfusion samples, reflecting persistence for at least 48 hours from previous infusion on those days. PR, partial response; SD, stable disease.
Response assessment using positron emission tomography/computed tomography scan was performed at 1, 3, and 6 months. Between the initial leukapheresis and RNA CART19 infusion, patients were allowed to undergo bridging chemotherapy at the discretion of the treating physician.
The primary objective of this pilot study was to determine manufacturing feasibility of nonviral RNA CART19 therapy and its safety in patients with cHL. Secondary objectives included response rates and survival. We also studied persistence of CART19 RNA expression as described previously.6
This study was approved by institutional review boards at the University of Pennsylvania and Children’s Hospital of Philadelphia.
Results and discussion
We enrolled 5 heavily pretreated patients with cHL with characteristics described in Table 1. All infused patients had a history of progression after cyclophosphamide.
Patient characteristics
| Patient N . | Age, y . | Prior lines of therapy, N . | Prior SCT . | Prior PD1 inhibitor . | Bridging therapy . |
|---|---|---|---|---|---|
| 1 | 41 | 8 | Auto | Yes | No |
| 2 | 22 | 5 | Auto | Yes | Yes |
| 3 | 42 | 5 | Auto | Yes | Yes |
| 4 | 24 | 9 | Auto/allo | No | No |
| 5 | 21 | 4 | NA | No | Yes |
| Patient N . | Age, y . | Prior lines of therapy, N . | Prior SCT . | Prior PD1 inhibitor . | Bridging therapy . |
|---|---|---|---|---|---|
| 1 | 41 | 8 | Auto | Yes | No |
| 2 | 22 | 5 | Auto | Yes | Yes |
| 3 | 42 | 5 | Auto | Yes | Yes |
| 4 | 24 | 9 | Auto/allo | No | No |
| 5 | 21 | 4 | NA | No | Yes |
allo, allogeneic; auto, autologous; NA, not applicable; SCT, stem-cell transplantation.
All 5 patients had successful manufacturing of nonviral RNA CART19 (Table 2). Patient 3 was taken off study before CART19 infusion because of new diagnosis of myelodysplastic syndrome. Four patients were infused with protocol-specified CART19 dose and were evaluable.
Manufacturing information and clinical outcomes
| Patient N . | CD3+CD45+ T cells in harvested product, %* . | Viability postthaw (first dose), %† . | CAR+ T cells in product, %‡ . | Total cells per kg per dose infused§ . | Doses infused, N . | Response, mo . | Alive . | |
|---|---|---|---|---|---|---|---|---|
| 1 . | 3 . | |||||||
| 1 | 96.6 | 97.2 | 71 | 2.11 × 106 | 6 | PD | — | No |
| 2 | 80.1 | 97.1 | 97.5 | 7.46 × 105 | 6 | SD | PD | Yes |
| 3 | 80.3 | NA | NA | NA | NA | NA | NA | Yes |
| 4 | 80.6 | 92.7 | 99 | 1.52 × 106 | 6 | CR | PD | Yes |
| 5 | 93.5 | 94.4 | 98.3 | 1.23 × 106 | 6 | PR | — | Yes |
| Patient N . | CD3+CD45+ T cells in harvested product, %* . | Viability postthaw (first dose), %† . | CAR+ T cells in product, %‡ . | Total cells per kg per dose infused§ . | Doses infused, N . | Response, mo . | Alive . | |
|---|---|---|---|---|---|---|---|---|
| 1 . | 3 . | |||||||
| 1 | 96.6 | 97.2 | 71 | 2.11 × 106 | 6 | PD | — | No |
| 2 | 80.1 | 97.1 | 97.5 | 7.46 × 105 | 6 | SD | PD | Yes |
| 3 | 80.3 | NA | NA | NA | NA | NA | NA | Yes |
| 4 | 80.6 | 92.7 | 99 | 1.52 × 106 | 6 | CR | PD | Yes |
| 5 | 93.5 | 94.4 | 98.3 | 1.23 × 106 | 6 | PR | — | Yes |
CR, complete response; NA, not applicable; PD, progressive disease; PR, partial response; SD, stable disease.
Release specification set at 80%.
Release specification set at 70%.
Release specification was set at 10%.
For patients weighing <80 kg (patients 1 and 4), the protocol-specified dose was 8 × 105 to 1.5 × 106 CAR+ cells per kg per dose, and for patients ≥80 kg (patients 2 and 5), the dose was 1 × 108 CAR+ cells per dose (±20%).
We collected blood samples before and 2 hours after each infusion and on days 21 and 28. CART19 RNA levels were measured using semiquantitative reverse transcription polymerase chain reaction and expressed as normalized ratio of 4-1BB/CD3ζ gene transcript copies (Figure 1B). CART19 RNA was detected in 80% of all postinfusion samples and in 20% of preinfusion samples. There was no CART19 RNA detected on day 21 or 28. This was consistent with the transient nature of nonviral CART19 cells.
The therapy was well tolerated. Hematological toxicities of this treatment were as expected and attributable primarily to the lymphodepleting chemotherapy. There were no grade 3 or 4 nonhematological toxicities. The most common adverse events reported were fatigue in 75% (2 with grade 1 and 1 with grade 2), grade 1 headache in 75%, and grade 1 confusion in 50% of patients. These were all transient and did not require any intervention. There was no cytokine release syndrome by clinical or laboratory criteria (eg, no interleukin-6 surge).
At the 1-month response assessment, patient 4 was found to have CR, patient 5 had PR, patient 2 had SD, and patient 1 had PD. Patient 4 had a history of both autologous and allogeneic stem-cell transplantation. He was enrolled after progression during gemcitabine monotherapy and did not receive bridging treatment. After achieving metabolic CR, he had PD at 3 months. There was no flare-up of graft-versus-host disease. He subsequently received an anti-PD1 antibody and is currently in CR. Patient 5 was enrolled after progression during multiagent salvage chemotherapy and received bridging therapy with brentuximab. Of note, he was previously refractory to brentuximab, but his other options were limited. Per protocol, he underwent reimaging after bridging brentuximab just before CART19 therapy and was found to have persistent disease. After achieving PR after CART19 infusions, he was taken off trial and treated with an anti-PD1 antibody and subsequently underwent autologous stem-cell transplantation and is in CR.
We compared CART19 RNA levels and persistence among the patients (Figure 1B). Patient 1 with PD had the lowest CART19 RNA levels and no evidence of persistence of CART19 RNA in any preinfusion samples. Patient 4, who achieved CR, had CART19 RNA detected in 3 of the 5 preinfusion samples, suggesting that longer persistence of CART19 may be associated with response.
Advances in cellular therapies for cHL have been slower when compared with non-Hodgkin lymphomas. Cellular therapies targeting CD30 or EBV proteins on HRS cells resulted in clinical responses in small studies.9-12 However, the immunosuppressive nature of the TME, including PD-L1/2 and PD1 interactions, could cause challenges for targeting HRS cells directly.13 We reasoned that by targeting HRS cells indirectly we could overcome these issues. Eliminating nonmalignant B lymphocytes from the TME may disrupt interactions essential for survival of HRS cells.14,15 CART19 may also eradicate clonotypic B cells, which have been found in peripheral blood of patients with cHL.5 These circulating CD19+ B cells have identical immunoglobulin H rearrangements to those found in the HRS cells and have been postulated to be HRS progenitors. Previous attempts to target peritumoral B cells in cHL with the anti-CD20 monoclonal antibody rituximab showed only modest clinical activity as monotherapy and no benefit when added to chemotherapy.16,17 However, using CART19 to target CD19 may have the potential advantage of affecting a broader range of B cells and also generating local cytokine release within the involved lymph nodes. Blood cytokine levels were measured, but these were difficult to interpret because of the possibility that they might not reflect changes within the TME and because of the small number of patients studied. Interestingly, CART19 therapy seems to have activity in some patients with multiple myeloma, which is another CD19− malignancy of B-cell origin.18,19 Ultimately, CAR-modified T cells with dual targeting (eg, CD30 and CD19), PD1-deficient CAR cells, or combinations of CAR cells with immune checkpoint inhibitors may be interesting strategies in cHL.
Our pilot study showed that collection of autologous T cells from patients with cHL and manufacturing of nonviral RNA CART19 are feasible. CART19 RNA was detected in peripheral blood for at least 48 hours after infusion in some patients, but not after 7 days. Although it is not possible to make any firm conclusions about clinical activity from this limited patient sample, the therapy seemed safe and resulted in some transient responses. On the basis of these findings, we are designing a lentiviral-transduced CART19 trial, capable of greater in vivo expansion and longer persistence, for patients with relapsed or refractory cHL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Lauren Strelec, Naseem Kerr, Katherine T. Marcucci, Kim-Marie Shea, Aliza Schmidt, Lester Lledo, and Amy Marshall of the Center for Cellular Immunotherapies and Clinical Cell and Vaccine Production Facility for cell manufacturing and testing and for clinical research assistance. The authors also thank Don Siegel and Nicole Aqui of the Hospital of the University of Pennsylvania for their help with apheresis.
This work was supported by funds from the Siegel Family Foundation, A. R. Spector Foundation, and Leukemia Lymphoma Society.
Authorship
Contribution: J.S. performed research, analyzed data, and wrote the paper; S.R.R. designed the study, performed research, analyzed data, and contributed to manuscript writing; S.I.G. designed the study, performed laboratory studies, analyzed data, and contributed to manuscript writing; S.A.G. designed the study; S.F.L., I.K., and J.J.M. performed laboratory studies; M.M.S. performed laboratory studies and analyzed manufacturing data; B.L. collected and analyzed data; A.R.M., S.D.N., and D.J.L. performed research and contributed to manuscript writing; M.R.Y. collected and analyzed data and prepared figures; B.L.L. designed the study and contributed to manufacturing; D.L.P. designed the study; C.H.J. designed the study and contributed to manufacturing; and S.J.S. designed the study, performed research, analyzed data, and contributed to manuscript writing.
Conflict-of-interest disclosure: J.S. received research support from Merck, Bristol-Myers Squibb, Seattle Genetics, Celgene, and Pharmacyclics and honoraria for consultancy from Kite, Seattle Genetics, and Bristol-Myers Squibb; S.I.G. received research support from Novartis and has patents/royalties with Novartis; S.A.G. received honoraria for consultancy from Adaptimmune, Jazz Pharmaceuticals, and Novartis; S.F.L. received research support from Novartis and honoraria from Genentech; J.J.M. received research support from Novartis; A.R.M. received research support from Regeneron, Portola, DTRM, Pharmacyclics, AbbVie, Acerta, and TG Therapeutics and honoraria for consultancy or advisory boards from Celgene, Kite, AbbVie, Gilead Sciences, Janssen, and TG Therapeutics; S.D.N. received research support from Takeda, Incyte, and Immunogen; D.J.L. received research support from Takeda and Curis and honoraria for consultancy from Curis; B.L.L. received research support from Novartis and honoraria for consultancy from Brammer Bio and GE Healthcare and has patents/royalties with Novartis and equity ownership in Tmunity Therapeutics; D.L.P. has family member employment/stock ownership with Genentech/Roche, received research support from Novartis and honoraria from Servier, Incyte, Novartis, and Immunovative Therapies, and has patents/royalties with Novartis; C.H.J. received research funding from Novartis and Tmunity Therapeutics and honoraria from Novartis, Celldex, Immune Design, and Copernicus Group, has patents/royalties with Novartis, and has equity ownership in Tmunity Therapeutics and Immune Design; S.J.S. received research funding from Celgene, Novartis, Bristol-Myers Squibb, Gilead, and Merck and honoraria for consultancy from Genentech, Celgene, Nordic Nanovector, Bristol-Myers Squibb, Seattle Genetics, Gilead, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Jakub Svoboda, Perelman Center for Advanced Medicine, University of Pennsylvania, 12th Floor, South Pavilion, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: jakub.svoboda@uphs.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal