In this issue of Blood, Wu et al report that following traumatic brain injury (TBI) von Willebrand Factor (VWF) is released, binds to microvesicles, and results in vascular leakage and coagulopathy, findings that were reversed by a disintegrin and metalloprotease with thrombospondin (ADAMTS-13).1
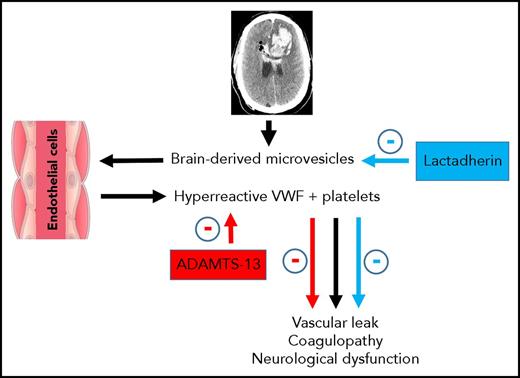
Overview of the pathway for VWF and brain-derived microparticle-induced vascular leakage and coagulopathy with reversal by ADAMTS-13. Following TBI, mivrovesicles are released from the injured brain and bind to hyperreactive VWF released from endothelial cells. In the presence of activated platelets, VWF-bound microvesicles result in vascular leakage, coagulopathy, and neurological dysfunction. These pathologic processes can be reversed by either ADAMTS-13, which blocks VWF reactivity, or lactadherin, which promotes clearance of the microvesicles.
Overview of the pathway for VWF and brain-derived microparticle-induced vascular leakage and coagulopathy with reversal by ADAMTS-13. Following TBI, mivrovesicles are released from the injured brain and bind to hyperreactive VWF released from endothelial cells. In the presence of activated platelets, VWF-bound microvesicles result in vascular leakage, coagulopathy, and neurological dysfunction. These pathologic processes can be reversed by either ADAMTS-13, which blocks VWF reactivity, or lactadherin, which promotes clearance of the microvesicles.
In the United States, TBI is a leading cause of death among children and adults.2 Each year, ∼1.5 million Americans sustain a TBI, with a mortality as high as one-third in patients with severe TBI.3 Additionally, when coagulopathy accompanies severe TBI, it is an independent predictor of poor prognosis.4,5 The pathophysiological mechanisms of coagulopathy are poorly defined and not well understood. Both hyper- and hypocoagulable states have been identified, each leading to opposite but deleterious outcomes. A hypercoagulable state can lead to brain ischemia from microthrombosis while hypocoagulopathy can result in hemorrhage with worsening of the brain injury. Coagulopathy had been attributed to release of tissue factor from the injured brain, but it is now understood that the mechanisms are much more complex. The authors previously provided novel insight into this complex pathway and reported that brain-derived microparticles are released from the injured brain into the systemic circulation and induce an early hypercoagulable state that is quickly followed by consumptive coagulopathy.6
In the current article, the authors hypothesized that VWF promotes TBI-induced and microvesicle-mediated blood-brain disruption and coagulopathy and that ADAMTS-13 protects endothelial integrity and prevents TBI-induced consumptive coagulopathy (see figure). VWF is an adhesive glycoprotein synthesized in megakaryocytes and endothelial cells and plays a role in hemostasis by recruiting platelets to the site of vessel injury.7 It is stored in Weibel-Palade bodies in endothelial cells and in α granules of platelets and is released into the circulation following trauma. The activity of VWF is modulated by ADAMTS-13, a metalloprotease that cleaves the large procoagulant VWF multimers once released into the circulation.8 Although low levels of VWF result in a bleeding diathesis, deficiency of ADAMTS-13 is associated with occlusive diseases such as myocardial infarction and stroke.
The authors investigated the protective effects of exogenously administered ADAMTS-13. To do so, they used their fluid percussive mouse brain injury model to confirm both brain and distant organ (lung) vascular leakage and bleeding as well coagulopathy following injury. They then demonstrated increased activity and adhesive levels of circulating VWF. In vitro, they were able to verify their previous findings that brain-derived microvesicles were responsible for vascular leakage by adding the microvesicle fraction of plasma from brain-injured mice to cultured endothelial cells. To assess the role of VWF, they added blocking antibody, and leakage was reduced. Interestingly, purified VWF in the absence of microvesicles had no effect on leakage.
When recombinant ADAMTS-13 (rADAMTS-13) was administered prior to injury, not only were vascular leakage and coagulopathy reduced, but neurological function and survival were improved. Similarly, ADAMTS-13−/− mice preconditioned with rADAMTS-13 displayed comparable protection. Perhaps the most clinically significant finding was the therapeutic efficacy of ADAMTS-13 administered postinjury. Mice receiving rADAMTS-13 30 minutes after injury also had reduced VWF adhesive activity as well as less cerebral vascular leakage and improved neurological function and survival.
The novel finding that VWF-bound microparticles may be responsible for enhanced vascular leakage and coagulopathy provides insight into the pathologic mechanisms responsible for secondary brain injury. Importantly, ADAMTS-13 may represent a potential therapeutic, although many questions remain unanswered. Verifying findings of this preclinical study in brain-injured patients may be an important next step.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal