In this issue of Blood, Fink et al report that a single amino acid change in cereblon (CRBN), the direct target of thalidomide and its derivatives, confers sensitivity to immunomodulatory drugs in mice.1
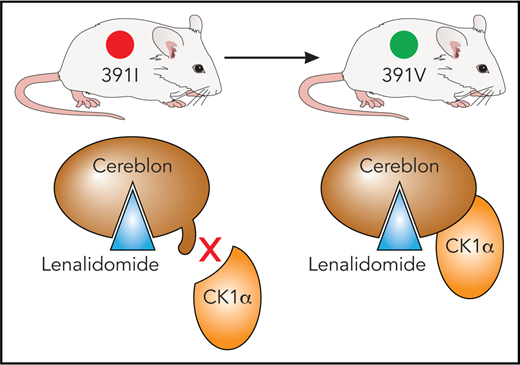
In mice, an isoleucine residue in cereblon may sterically clash with substrate proteins, including CK1α. By using a single I391V amino acid change, Fink et al engineered a mouse model sensitive to the effects of immunomodulatory drugs. Professional illustration by Patrick Lane, ScEYEnce Studios, based on a sketch by James Sanchez, City of Hope.
In mice, an isoleucine residue in cereblon may sterically clash with substrate proteins, including CK1α. By using a single I391V amino acid change, Fink et al engineered a mouse model sensitive to the effects of immunomodulatory drugs. Professional illustration by Patrick Lane, ScEYEnce Studios, based on a sketch by James Sanchez, City of Hope.
Few drugs have experienced a comeback equal to that of thalidomide. It was a purported sedative that was withdrawn from the market when its teratogenic effects were learned of. Thalidomide has now found a new role as an immunomodulatory drug for multiple myeloma (MM). But all comebacks are finite, and thalidomide has largely been succeeded by its derivatives lenalidomide and pomalidomide, which have greater potency and fewer adverse effects.
Lenalidomide shows efficacy for mantle cell lymphoma in addition to MM, and it has particularly strong activity against myelodysplastic syndrome (MDS) with deletion of chromosome 5q (del5(q)), yielding a complete cytogenetic remission of about 50%.2,3 However, research on immunomodulatory drugs has been hindered by the fact that they are inactive in rodent models.4 It had been discovered that an isoleucine residue in murine CRBN, although it does not block binding of immunomodulatory drugs to CRBN, leads to a steric clash between CRBN and its substrate proteins.5 A single amino acid change to the corresponding human amino acid valine (ie, I391V) permits lenalidomide-dependent degradation of casein kinase 1α (CK1α) in human del(5q) MDS. CK1α is a negative regulator of p536 and exhibits haploinsufficient expression in del(5q) MDS,7 possibly explaining the therapeutic window of lenalidomide in this disease.
The development of a mouse model with this I391V amino acid change in CRBN (CRBNI391V) has been reported by Fink et al. The authors confirmed that lenalidomide- and pomalidomide-treated cells from this mouse strain exhibit a decrease in known substrate proteins such as Ikzf1 and Zfp91. They also confirmed in vitro that T cells isolated from both homozygous and heterozygous mutant mice exhibit increased production of interleukin-2 upon treatment with lenalidomide or pomalidomide, but the T-cell contribution to the anticancer efficacy of this class of drug still remains an open question. Importantly, degradation of substrate proteins occurred similarly in vivo by administration of thalidomide derivatives. However, elevated doses were necessary (eg, 50 mg/kg lenalidomide) to achieve maximum effect. A higher rate of metabolism of these drugs in mice may account for the need for high doses. It is unclear how this finding will affect the clinical relevance of the model.
The authors also explored the effects of lenalidomide on hematopoietic cells, and they found a decline in total white blood cell count, a drop in platelet counts, and other effects on native hematopoiesis. The availability of a murine model for studying the toxicity and long-term impact of immunomodulatory drugs is valuable (while considering the issue of high dosing), especially because these drugs may be used for extended periods of time in patients, either as maintenance therapy or possibly in precursor conditions such as smoldering MM.
In addition, the researchers used their CRBNI391V model to demonstrate the role of lenalidomide in degrading CK1α (see figure) and its therapeutic efficacy associated with the presence of an intact Tp53 gene, because heterozygous deletion of Tp53 was sufficient to cause lenalidomide resistance in Csnk1α1+/−CrbnI391V mice. Interestingly, pomalidomide and other immunomodulatory drugs tested do not affect CK1α expression,5 supporting the idea that subtle differences in immunomodulatory drug binding to CRBN may affect substrate preference as well as providing a rationale for the production of next-generation immunomodulatory drugs.
Finally, mouse models created to help us understand the genesis of thalidomide-induced limb defects may also help us understand therapeutic effects, because CRBN was, in fact, identified in a study of the target of thalidomide teratogenicity.8 Lenalidomide causes limb defects in monkeys but has no such effects in mice.9 Is the murine version of CRBN behind the difference? The authors treated CrbnI391V/I391V mice with thalidomide or lenalidomide and, although a high rate of fetal loss was reported, they noted that litters carried to term generally had normal morphology. The authors state that “additional species-specific differences in the sequence of downstream targets, the phenotypic consequences of their degradation, or their dependence on Crbn residues other than I391V” may explain the absence of certain birth defects.
Indeed, Eichner et al10 report that immunomodulatory drugs outcompete CRBN for binding to CD147 and MCT1, a complex that promotes biological functions including angiogenesis and lactate export. This disruption leads to the destabilization of the CD147/MCT1 complex. Immunomodulatory drugs had no effect on the protein levels of CD147/MCT1 in mouse cells.10 Conversely, in zebrafish, which are sensitive to the teratogenic effects of thalidomide, induced loss of CD147 phenocopied these effects.10 For both toxicity and efficacy studies, it is important to assess whether CrbnI391V mice are sensitive to immunomodulatory drug-induced destabilization of CD147/MCT1 and, if not, which further mutations (in CRBN or elsewhere) would sensitize mice. Nevertheless, the CrbnI381V mice introduced in the article by Fink et al represent a significant first step in allowing immunomodulatory drug research in a mouse model and should help address a number of questions with respect to mechanism of action and toxicity.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal