In this issue of Blood, Miao et al1 have investigated the role of brain iron accumulation and silent cerebral infarction (SCI) in sickle cell disease (SCD), as well as considered whether these pathologies might be connected. Although iron deposition may play a crucial role in organ damage in SCD, the mechanisms involved are unclear, and there are few studies in the brain. To investigate further, Miao et al exploited the emerging technique of magnetic resonance imaging (MRI) quantitative susceptibility mapping (QSM), which provides a measure of tissue magnetic susceptibility that is strongly correlated with its iron content. They report QSM evidence for iron accumulation in the putamen, substantia nigra, and red nucleus in adolescent and young adult patients with SCD compared with controls. They also found significantly higher susceptibility in the globus pallidus and substantia nigra of SCD patients with SCI. When combining SCD patients with controls, they found a significant increase in susceptibility with age in the substantia nigra (see figure) and other deep gray matter regions, as originally documented histologically and confirmed in R2* and QSM MRI studies in healthy people.
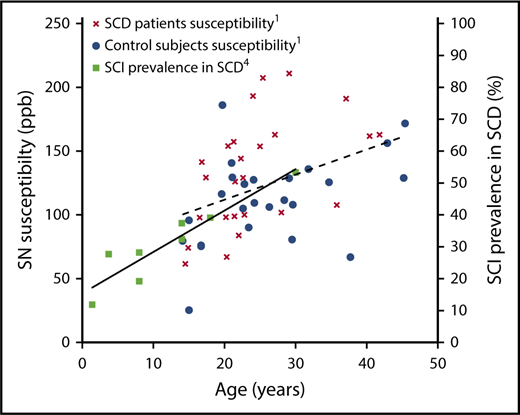
Susceptibility plotted against age in the substantia nigra (SN) of SCD patients and controls from supplemental Figure 2 in the article by Miao et al that begins on page 1618 with the best-fit line (broken; left y-axis). Cumulative prevalence of silent cerebral infarction with age in children and young adults with SCD from Figure 1 in the article by Kassim et al4 with the best-fit line (unbroken; right y-axis). Courtesy of Xin Miao, John C. Wood, and Mark Rodeghier.
Susceptibility plotted against age in the substantia nigra (SN) of SCD patients and controls from supplemental Figure 2 in the article by Miao et al that begins on page 1618 with the best-fit line (broken; left y-axis). Cumulative prevalence of silent cerebral infarction with age in children and young adults with SCD from Figure 1 in the article by Kassim et al4 with the best-fit line (unbroken; right y-axis). Courtesy of Xin Miao, John C. Wood, and Mark Rodeghier.
The effect of anemia on brain iron has received little attention, and it is of interest that there was a significant negative correlation between hemoglobin levels and susceptibility when both SCD and healthy subjects were considered. It is possible that this is driven by increased levels of (high susceptibility) deoxyhemoglobin in hypoxic regions in anemic patients. Anemic hypoxia might even have a role in determining iron accumulation over the lifespan, perhaps as part of a homeostatic mechanism driving improvement in tissue oxygenation. Even for patients on chronic transfusion regimes, brain susceptibility was not associated with somatic iron status, providing reassurance that iron overload in the body does not appear to result in brain iron accumulation, although larger studies are warranted. There was no difference in susceptibility in controls with HbAA and HbAS genotypes, so these groups were combined, but comparisons could not be made among those with HbSS, HbSβ0thalassemia, and HbSC genotypes in the relatively small number of patients.
As the authors point out, excessive brain iron could accelerate neurodegeneration in SCD, as has been shown in other conditions, such as Parkinson disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, and Alzheimer disease.2 There are relatively few studies of neurological symptoms and signs in adults with SCD associated with MRI findings. However, the extrapyramidal movement disorders typical of Parkinson disease and the progressive paralysis of ALS have not been documented in SCD in articles cited in PubMed and appear to be rare in London (1 case across the 5 centers seeing large numbers of adults; Jo Howard, Guy's Hospital, Paul Telfer, Royal London Hospital, Anne Yardumian, North Middlesex Hospital, Elizabeth Rhodes, St. George's Hospital, and Josu de la Fuente, St. Mary's Hospital, oral communication, 8 August 2018) and the United States (0 cases reported in 1 large center; Lori Jordan, Vanderbilt University, oral communication, 8 August 2018). In addition, although demyelination similar to that seen in multiple sclerosis is a candidate to explain the white matter abnormalities seen on diffusion tensor imaging3 as well as T2-weighted imaging, the current paradigm is that these are SCI,4 and the focus has been on the association with stroke risk.
The authors suggest a link between iron accumulation and SCI in SCD patients; they found significantly higher susceptibility in the globus pallidus and substantia nigra of SCD patients with SCI. However, it is not clear what the mechanism linking basal ganglia iron accumulation to white matter damage (SCI) might be. The accumulation of SCI starts very early (see figure),4 before the trajectory of the iron accumulation in SCD appears to diverge from that seen in controls (Russell Murdoch, University College London, oral communication, 6 August 2018). In 1 cited study in children with SCD,5 and in another looking at adults with β-thalassemia,6 who also accumulate SCI, brain iron on QSM was actually significantly lower in the globus pallidus of SCD patients than in controls. This suggests that the basal ganglia and white matter pathologies may not be causally linked but may both be related to another factor, such as hypoxic exposure.3 The data of Miao et al and Qiu et al7 in SCD suggest iron accumulation in the choroid plexus, the epithelial cells of which appear to control iron delivery into the cerebrospinal fluid.8 This does, however, make it difficult to reference the susceptibility values to cerebrospinal fluid, so Miao et al chose to reference their deep gray matter values to the splenium of the corpus callosum. This white matter structure returns an abnormal diffusion tensor imaging signal in SCD patients in association with low oxygen saturation.3 It is important to note that QSM reflects tissue components of both positive and negative susceptibility (eg, calcifications and myelin) in addition to iron and deoxyhemoglobin. These complexities illustrate how difficult it may be to tease apart the mechanisms at play here.
The key question is whether iron accumulation triggers cognitive decline in SCD. There is some QSM evidence for an association of increased brain iron in the hippocampus and temporal and frontal lobes with the accumulation of amyloid-β plaques and cognitive decline in Alzheimer disease.9 However, there are as yet no QSM, pathological, or positron emission tomography9 studies showing evidence that pathology involving amyloid-β deposition underlies cognitive difficulties in SCD. In fact, pediatric studies have demonstrated that susceptibility in the caudate nucleus is positively correlated with cognitive function, including working memory and spatial IQ,10 suggesting that higher brain iron levels are associated with better cognition in children in the general population, although there are no published data in SCD. To better understand the importance of iron accumulation in development and cognitive decline, it will be important to investigate susceptibility, in addition to the basal ganglia nuclei and cerebellum investigated here, in the deep white matter, hippocampus, frontal, temporal, parietal, and occipital lobes, ideally in parallel with other neuroimaging techniques looking at cerebral hemodynamics and tissue structure. Studies over small age ranges may be required, as in some of these areas, the effect of age is definitely nonlinear and may not even be monotonic.
If brain iron accumulation does turn out to be causally associated with cognitive decline in older patients with SCD, then we will need to fully understand the mechanisms to be able to prevent this. The role of chelating agents could be considered, although these have not yet proved beneficial in diseases such as Parkinson disease. We look forward to further imaging studies to clarify the role and mechanisms linking anemia, hypoxia, tissue damage/SCI, and brain iron accumulation in SCD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal