In this issue of Blood, Haddad and colleagues on behalf of the Primary Immune Deficiency Treatment Consortium report retrospective outcome data on hematopoietic stem cell transplantation (HSCT) for 662 patients with severe combined immunodeficiency (SCID) treated between 1982 and 2012 in 33 North American institutions.1
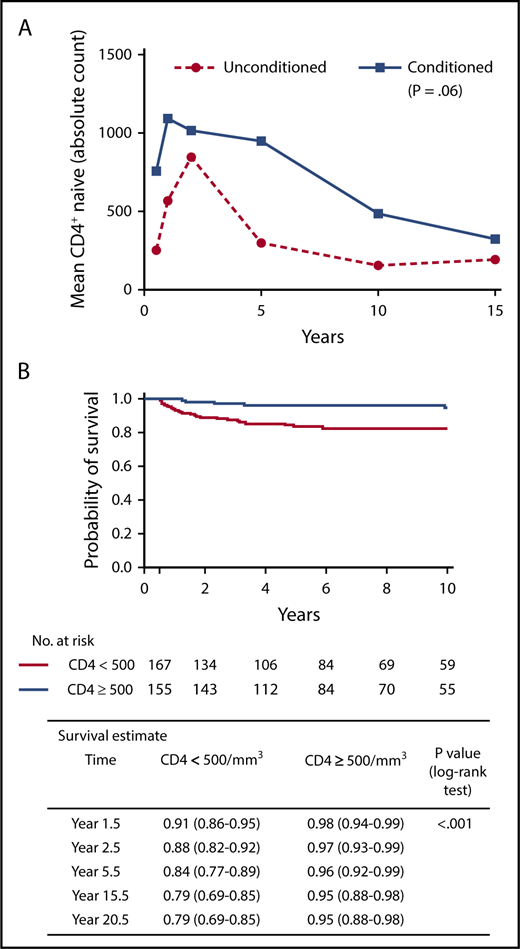
(A) Longitudinal increased numbers of naive T lymphocytes are associated with chemotherapy conditioning. Adapted from Abd Hamid et al.4 (B) Overall long-term survival is associated with increased numbers of naive T lymphocytes. Panel (B) has been adapted from Figure 3A in the article by Haddad et al that begins on page 1737.
(A) Longitudinal increased numbers of naive T lymphocytes are associated with chemotherapy conditioning. Adapted from Abd Hamid et al.4 (B) Overall long-term survival is associated with increased numbers of naive T lymphocytes. Panel (B) has been adapted from Figure 3A in the article by Haddad et al that begins on page 1737.
Inborn errors of immunity are genetic diseases predisposing affected individuals to an increased risk of infection, autoimmunity, or malignancy. The most severe of these disorders, SCID, characterized by absent or dysfunctional T lymphocytes affecting cellular and humoral adaptive immunity, is life threatening when recognized too late. Contingent on the genetic defect, B lymphocytes and natural killer cells may be present or absent. Since the first HSCT for SCID was performed 50 years ago, discussion among specialists caring for these patients has continued over the best approach to treatment. Following that first report, much has been learned about different SCID genotypes, clinical and immunophenotypes, and natural history. In parallel, transplant techniques and approaches have evolved so that today, survival in this group of infants approaches 90% in recent series.2,3 However, debate around the best alternative donor, use of conditioning, and optimum early biomarkers indicating requirement for a further transplant procedure continues. Problems in resolving the issues include rarity of the disease, and limited experience of any 1 center in treating these patients. Furthermore, historic series have analyzed SCID on phenotypic presentation, whereas genotype, rather than phenotype, may be more important.4,5 In this issue, Haddad and colleagues analyze outcome of 662 SCID patients treated between 1982 and 2012 in 33 North American institutions. This is one of the largest multicenter studies to date, and for the first time in such a cohort, outcome has been documented according to genotype. As with any such study, a number of limitations are apparent: over the timescale of 30 years, there have been tremendous improvements in approach to diagnosis of SCID and all aspects of HSCT. The multicenter approach means it is inevitable that data are missing, and not all patients will be adequately accounted for. Centers may use different transplant techniques and specialize in treating specific genetic defects. Furthermore, genetic information was incomplete and only available in 58% of patients. Nevertheless, given the rigorous eligibility criteria for the study, important information can be gleaned.
First, the superiority of matched sibling donors corroborates results from previous studies. However, there were no significant differences in survival when comparing other donor types, which confirms observations from the European Inborn Errors Working Party in their most recent epoch analysis, and others.3,6 This information will be useful for resource-limited centers for which use of unrelated donors may be precluded. Fine detail about differences in T-lymphocyte depletion methods was not available, and in the modern era, nuances in technique may improve survival further. Second, the importance of transplantation before infection is present is now well established,2 but the current study noted outcome differences in age at transplant in those with infection, with patients >3.5 months of age at transplant having a worse outcome than those transplanted <3.5 months of age. The reasons for this are not clear and may include more advanced end-organ damage in the older group, but emphasize that SCID is a medical emergency, and early diagnosis through newborn screening and early transplant lead to best outcomes.7
Third, this study found no difference in transplant outcome between patients with “classical” or atypical/leaky SCID, in contradistinction to the results reported by the European group.3 The reasons for this difference in outcome are not obvious, and detailed analysis of the 2 groups may be required to explain this difference. Fourth, similar to the European group, this study found no survival difference between those receiving chemotherapy conditioning and those receiving only immunosuppression or no preparative regimen. Although at first reading this might argue that either approach is valid, the present study also confirmed observations that a pretransplant preparative regimen is associated with durable engraftment,8 the primary goal of treatment of these patients. Thus, this study adds to the growing body of evidence that suggests best outcomes in terms of graft durability and immune reconstitution occur when a chemotherapy-based preparative regimen is administered before allograft infusion. However, the importance of detailed analysis based on genotype is clearly demonstrated by the finding that survival of patients with radiosensitive SCID (Artemis deficiency) was worse than those of RAG-deficient SCID, despite similar immunotypes, and not demonstrated in a previous report.9 Importantly, the increased mortality in the radiosensitive SCID group was not due to infectious causes, suggesting that the interplay of chemotherapy and the systemic nature of the radiosensitive defect may play an important role in outcome, so although conditioning may be required to achieve enduring immunity, safer, less toxic methods of achieving robust stem cell engraftment are required.10
Finally and importantly, this study leads us closer to identifying failing grafts, to enable early and effective intervention. It is difficult to identify in which patients immune reconstitution is likely to be suboptimal, and therefore, in whom an early decision to boost or retransplant should be taken, important because early intervention is more likely to be successful. However, additional procedures should be avoided in those patients who do not require them. This large cohort study confirms and connects observations from a number of previous small studies, namely that durable T-lymphocyte reconstitution associates with better survival; good T-lymphocyte reconstitution at 1 to 2 years post-HSCT associates with better T-lymphocyte long-term immune reconstitution, and high T-lymphocyte receptor excision (TREC) circle counts, markers of thymopoiesis, at 6 months associate with robust long-term T-lymphocyte reconstitution (see figure).
This study and other large cohort studies of SCID patients strongly emphasize the critical importance of multicenter collaboration and long-term follow-up of these rare patients in experienced centers collecting good-quality data; without careful analysis of the minutiae of immune reconstitution according to preparative conditioning regimen and genotype over 30 years, this study would have no added value over previous studies. Over the years, small pieces of the jigsaw puzzle have been pieced together, so we now have a much clearer picture of how our treatments impact our patients in the short and long term. The next steps will be to gather good-quality data on the impact of our treatment on life quality, and on very long-term outcomes. This study helps lay the foundation for those future analyses.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal