Abstract
Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) is a recently described form of T-cell non-Hodgkin lymphoma now formally recognized by the World Health Organization classification of lymphoid neoplasms. The disease most often presents with a delayed seroma around the breast implant, almost exclusively with a textured surface, and manifests with breast pain, swelling or asymmetry, capsular contracture, but can also present with a breast mass, and lymph node involvement. The prognosis of BIA-ALCL is favorable compared with many other subtypes of systemic T-cell lymphoma; however, unlike other non-Hodgkin lymphomas, complete surgical excision for localized disease is an important part of the management of these patients. In this paper, we share our recommendations for a multidisciplinary team approach to the diagnosis, workup, and treatment of BIA-ALCL in line with consensus guidelines by the National Comprehensive Cancer Network.
Introduction
Anaplastic large cell lymphomas (ALCLs) are an uncommon group of T-cell non-Hodgkin lymphomas that universally express CD30. Systemic subtypes categorized by ALK expression (ALK positive and ALK negative) and the more indolent primary cutaneous ALCL are well described as histologically similar but clinically distinct entities.1 Breast implant–associated ALCL (BIA-ALCL) is more recently recognized and was provisionally classified in the 2016 revision of the World Health Organization classification of lymphoid neoplasms.2 Given the uncommon nature of this recently recognized malignancy, clinical decision making is informed primarily on retrospective series, expert opinions, and our own clinical experience and biases. Thus, this piece represents our multidisciplinary opinion regarding how we approach diagnosis and treatment of BIA-ALCL, which is similar to the guidance provided by the National Comprehensive Cancer Network (NCCN) guidelines.3
Epidemiology
The first case of BIA-ALCL was reported in 1997 when a 41-year old woman developed a CD30+ peripheral T-cell lymphoma mass most consistent with ALCL in the fibrous capsule surrounding her cosmetic textured-surface breast implant.4 More cases have emerged over the past 2 decades with a significant increase in known cases and public awareness following a 2011 Food and Drug Administration (FDA) safety advisory, leading to an international effort to better understand the novel entity.5 Implants may be categorized by their internal fill (eg, saline or silicone), shape (eg, contoured or round), and the outer surface shell (eg, smooth or textured). Some believe that textured surface implants have a reduced frequency malposition and capsular contracture, although this is not validated in a large prospective comparative trial. To date, cases (for which the implant surface was reported) have only been seen in textured implants with no cases seen in patients with a documented history of only smooth implants.6-9
Since 1997, more than 500 unique cases have been reported in 23 countries worldwide with most cases diagnosed in the setting of a delayed seroma manifesting as breast swelling and asymmetry (Figure 1). On average, the diagnosis is made over 7 to 10 years after implantation with a range of 2.2 months to 28 years from implantation.8,10 Although this article discusses our recommended approach to diagnosis and management of this rare entity, it is important to consider that there are at least 10 million women worldwide with implants, and ∼550 000 implants are placed per year in the United States for cosmetic and reconstructive indications.5 Based on 100 US cases of BIA-ALCL as of December 2016, it was estimated that the incidence of BIA-ALCL in the United States was 33 per 1 million persons with textured breast implants.10 This equates to an estimated lifetime risk of ∼1 in 30 000 for women with a textured implant. Similarly, other international series estimating lifetime risk range from 1:1000 to 1:10 000 patients with a textured implant.8,11-13 A recent population-based case-control study of the nationwide Dutch pathology registry reported a cumulative risk of 1:6920 women with textured implants.13 An FDA-mandated implant manufacturer prospective study of 17 656 women with 31 985 textured implants reported 6 BIA-ALCL cases.14 Although increasingly recognized with a greater professional and public awareness, BIA-ALCL remains an uncommon and emerging complication of breast implantation and requires a multidisciplinary approach.
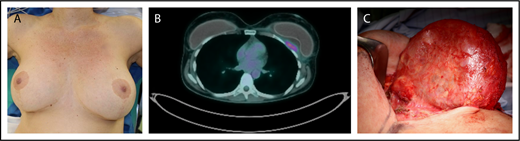
Clinical example of BIA-ALCL. Patient is a 42-year-old woman who presented with a late periprosthetic seroma of the left breast capsule (A) ∼7 years following cosmetic augmentation-mastopexy with bilateral textured breast implants. A fine needle aspirate (FNA) of the periprosthetic effusion demonstrated clonal expansion of CD30+ large anaplastic T cells. (B) A preoperative PET/CT scan demonstrated a posterior capsule wall mass invading the chest wall. (C) Specimen from a bilateral explantation; total capsulectomy with excision of the skin involvement demonstrated a posterior mass on the capsule. Complete surgical excision is essential as residual disease is associated with disease progression.
Clinical example of BIA-ALCL. Patient is a 42-year-old woman who presented with a late periprosthetic seroma of the left breast capsule (A) ∼7 years following cosmetic augmentation-mastopexy with bilateral textured breast implants. A fine needle aspirate (FNA) of the periprosthetic effusion demonstrated clonal expansion of CD30+ large anaplastic T cells. (B) A preoperative PET/CT scan demonstrated a posterior capsule wall mass invading the chest wall. (C) Specimen from a bilateral explantation; total capsulectomy with excision of the skin involvement demonstrated a posterior mass on the capsule. Complete surgical excision is essential as residual disease is associated with disease progression.
Case 1
A 43-year-old woman with a history of bilateral textured silicone implant placed 10 years ago presentd to her breast surgeon with swelling in her left breast. She noted that the swelling began 3 weeks ago and had been increasing and now is associated with tenderness without erythema or fevers. She denied trauma to the breast or recent accidents. Her examination was notable for asymmetry of the breast without erythema, fluctuance, induration, or ecchymosis.
Case 2
A 68-year-old woman with a history of invasive ductal breast cancer (T3N2MX estrogen/progesterone receptor–positive, Her2 negative) 7 years ago presented with a palpable mass in the left breast for 4 weeks. At that time, she underwent left total mastectomy, adjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel and subsequent reconstruction. She remained on an aromatase inhibitor until 3 months ago. She was seen by her breast surgeon, who performed an ultrasound that showed a 300mL seroma surrounding the textured breast implant. Magnetic resonance imaging (MRI) of the breast showed a seroma surrounding the breast implant as well as a 1-cm mass on the capsule of the implant.
Clinical presentation
BIA-ALCL has been observed in women who have undergone breast implantation for cosmetic or reconstruction purposes. The median age of patients at the time of diagnosis is in the mid-50s with the median interval from implantation to diagnosis of BIA-ALCL being 7 to 10 years.6,7,15 Of women with BIA-ALCL, 60% to 80% present with a persistent seroma, which can be accompanied by breast swelling, asymmetry, or pain7 (Figure 1). Delayed seromas (ie, developing ≥1 year after implantation) occur in ∼0.05% to 0.1% of patients who receive textured breast implants, and among those with delayed seromas, the risk of BIA-ALCL is estimated at ∼10%.14,16-18 It is important to note that BIA-ALCL is a relatively rare complication of textured breast implants, and other more common causes of delayed seromas include external trauma and infection.
BIA-ALCL generally develops within a seroma around the implant and less commonly involves or invades the fibrous capsule (Figure 2). Axillary lymphadenopathy has been reported in up to 15% of cases, and ∼10% to 20% of patients present with a breast mass through the scar capsule around the implant.6,7 Cutaneous lesions (eg, erythema, cutaneous papules), capsular contractures, in addition to seroma and capsule involvement, and B symptoms have been reported at presentation but are far less common.6,7,19 Interestingly, patients with bilateral breast capsule involvement have been rarely reported.11,20
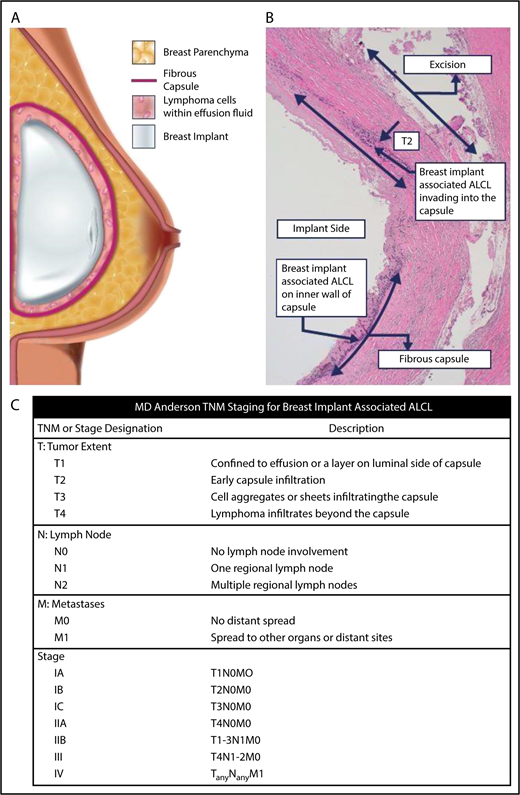
BIA-ALCL schematic and TNM staging. (A) The schematic demonstrates that BIA-ALCL typically presents in the seroma surrounding the breast implant. The lymphoma is usually contained within the fibrous capsule and distinct from breast parenchyma. BIA-ALCL typically is isolated to within the fluid and/or inner wall of the capsule, although invasion into or beyond the capsule is less commonly seen and associated with a worse prognosis. (B) This can be seen histologically or on gross review of the pathology at the time of surgery. (C) Clinical and pathologic staging of BIA-ALCL follows the MD Anderson Solid Tumor Staging System modeled after the American Joint Committee on Cancer TNM stages. Using this system, BIA-ALCL patients have a spectrum of disease from IA (35.6%, effusion only), IB (11.5%), IC (13.8%), IIA (25.3%), IIB (4.6%), III (9.2%), to stage IV (0%). Adapted from Clemens et al7 with permission.
BIA-ALCL schematic and TNM staging. (A) The schematic demonstrates that BIA-ALCL typically presents in the seroma surrounding the breast implant. The lymphoma is usually contained within the fibrous capsule and distinct from breast parenchyma. BIA-ALCL typically is isolated to within the fluid and/or inner wall of the capsule, although invasion into or beyond the capsule is less commonly seen and associated with a worse prognosis. (B) This can be seen histologically or on gross review of the pathology at the time of surgery. (C) Clinical and pathologic staging of BIA-ALCL follows the MD Anderson Solid Tumor Staging System modeled after the American Joint Committee on Cancer TNM stages. Using this system, BIA-ALCL patients have a spectrum of disease from IA (35.6%, effusion only), IB (11.5%), IC (13.8%), IIA (25.3%), IIB (4.6%), III (9.2%), to stage IV (0%). Adapted from Clemens et al7 with permission.
With regards to risk factors for BIA-ALCL, to date, cases have only been seen in textured devices or in cases where the implant type was not reported. No cases have been seen in patients with a documented history of only smooth implants.6-8 Although textured implants are overwhelmingly preferred by surgeons in Australia, Asia, Europe, and South America, they represent <13% of the US implant market at present.21 Three male to female transgender patients with BIA-ALCL have also been reported, including 1 unpublished case.22,23
Although the pathogenesis of BIA-ALCL remains unclear, there are a number of hypotheses regarding the risks of developing this malignancy. Textured implants appear to elicit a marked local T-cell immune response (both helper and cytotoxic) in comparison with smooth implants.24 Some hypothesize that the surface area of the implant, created by texture particulate on the implant surface, increases the risk of BIA-ALCL.25 In a series of 55 patients, Loch-Wilkinson et al hypothesized that among textured implants, those with higher surface area carry higher risk with inflammation triggering a T-cell CD30+ clonal expansion.6,26 Textured implants shed silicone particulate, whereas smooth implants do not. Macrophages digesting silicone particulate form foamy cells and secrete proinflammatory cytokines, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor, which in turn lead to T-cell chemotaxis and replication27,28 (Figure 3D). Some have found BIA-ALCL arising in the presence of an allergic inflammation cascade mediated by immunoglobulin E and IL-10.27 Others have demonstrated ubiquitous bacteria in normal and abnormal breast implant capsules and speculate that the lipopolysaccharide membrane of gram-negative bacteria in a biofilm may contribute to lymphocyte hyperplasia.29 These observations have led to the hypothesis that BIA-ALCL stems from an aberrant reactive T-cell lymphoproliferative population.26 Despite the concerted efforts of many, these factors have not yet been proven to be causal in the pathogenesis of BIA-ALCL or identified the necessary steps leading to transformation from inflammation to malignancy around a textured implant.29 Activating somatic mutations in the JAK-STAT pathway, and mutations in SOCS1, TP53, and DNMT3A are described in BIA-ALCL. Dual-activating mutations in JAK proteins and STAT3 have been described and carry similarities to systemic ALK-negative ALCL.30,31 The aberrancy in the JAK1/STAT3 pathway supports the model of pathogenesis, suggesting that similar driver mutations and molecular pathways exist between BIA-ALCL and systemic ALK-negative ALCL. Interestingly, germline mutations in the JAK3 pathway have been identified in patients with BIA-ALCL, which leads some to hypothesize that in some cases host factors may predispose to development of BIA-ALCL.
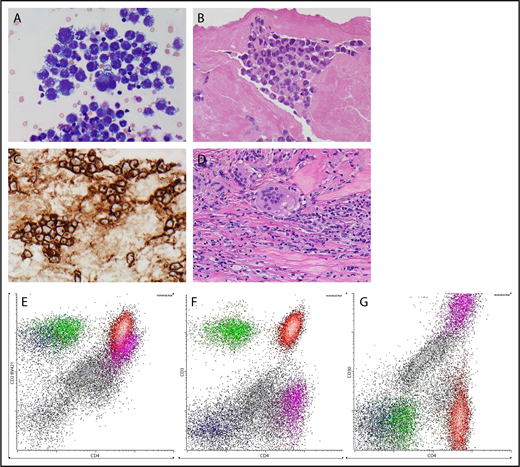
Pathology. (A) Cytological appearances of BIA-ALCL cells on cytopsin preparation from an effusion (original magnification ×600; Giemsa stain). (B) Histological appearances of BIA-ALCL in capsulectomy specimen (original magnification ×400; hematoxylin and eosin stain). (C) CD30 expression by the neoplastic cells of BIA-ALCL (original magnification ×400; immunoperoxidase stain). (D) Foreign body giant cells reaction against silicone particulate shed from textured implant in the capsule. (original magnification ×400; hematoxylin and eosin stain) (E) Flow cytometric immunophenotyping of effusion from a case of BIA-ALCL. The neoplastic cells, shown with purple dots, express CD4 and CD2 (dim) (E) but not CD3 (F). They are brightly positive for CD30. Normal CD4 and CD8-positive T cells are shown in red and green, respectively. Small numbers of NK cells, shown by dark blue dots, are present. The remaining mononuclear cells, shown in gray dots, are mostly macrophages. All panels are gated on mononuclear cells. (G) The flow cytometric gate for CD30.
Pathology. (A) Cytological appearances of BIA-ALCL cells on cytopsin preparation from an effusion (original magnification ×600; Giemsa stain). (B) Histological appearances of BIA-ALCL in capsulectomy specimen (original magnification ×400; hematoxylin and eosin stain). (C) CD30 expression by the neoplastic cells of BIA-ALCL (original magnification ×400; immunoperoxidase stain). (D) Foreign body giant cells reaction against silicone particulate shed from textured implant in the capsule. (original magnification ×400; hematoxylin and eosin stain) (E) Flow cytometric immunophenotyping of effusion from a case of BIA-ALCL. The neoplastic cells, shown with purple dots, express CD4 and CD2 (dim) (E) but not CD3 (F). They are brightly positive for CD30. Normal CD4 and CD8-positive T cells are shown in red and green, respectively. Small numbers of NK cells, shown by dark blue dots, are present. The remaining mononuclear cells, shown in gray dots, are mostly macrophages. All panels are gated on mononuclear cells. (G) The flow cytometric gate for CD30.
Evaluation of suspected cases
No screening, testing, or prophylactic surgery is recommended for asymptomatic patients beyond regular mammograms as part of standard breast cancer screening. Patients who have signs or symptoms of possible BIA-ALCL, particularly the development of a seroma more than a year after breast implantation, should undergo evaluation.3,32 Patients should undergo imaging with ultrasound or breast MRI to document the presence of fluid around the implant or a mass. Breast MRIs are particularly helpful in patients with suspicion for a mass. The presence of effusion or mass should prompt an aspiration of fluid or biopsy of mass. In the case of patients with an effusion, fine needle aspirate with cytology and flow cytometry (including evaluation of CD30) with specific instructions to evaluate for suspected BIA-ALCL is critical (see “Pathologic definition”). It is important to obtain as large a volume of fluid as possible because less optimal sampling may lead to delay in diagnosis or indeterminate results. For patients who present with a breast mass or lymphadenopathy, a biopsy of the mass or lymph node with concurrent flow cytometry and evaluation of T-cell markers including CD30 is important. Again alerting the pathologist of the suspicion for BIA-ALCL is helpful because specific markers are required for accurate diagnosis that would not typically be included in the more common workup of a breast mass. T-cell gene rearrangements can be performed to aid in the diagnosis; however, a subset of systemic ALCL does not demonstrate clonality by the T-cell receptor and conversely reactive populations of T cells can demonstrate a false positive small clonal population of T cells.26,33 A parenchymal breast mass, discrete from the capsule, is not typical of BIA-ALCL. If after pathology evaluation, diagnosis of lymphoma is indeterminate, close observation with a low threshold for repeating the biopsy or aspiration if sufficient fluid persists or recurs and secondary hematopathology consultation at a center with experience in diagnosis BIA-ALCL should be considered. There is no standard practice for patients with indeterminate diagnosis; however, clinical evaluation every 3 to 4 months by a breast or plastic surgeon is reasonable. If the pathology is negative, the patient should be referred to a plastic surgeon for management as a benign seroma. For those who present with suspicious lymphadenopathy, positron emission tomography–computed tomography (PET/CT) is useful to identify sites of disease most amenable to biopsy.
Pathologic definition
Most cases of BIA-ALCL present with effusions adjacent to the implant, and therefore, cytological examination of the FNA specimen is essential. Obtaining a larger volume of fluid (at least 10 mL but ideally most cases require >50 mL to obtain the correct diagnosis) and communication with pathology regarding a concern for BIA-ALCL are helpful to prevent delays in diagnosis. Examination of the specimen should include smears or cytospin preparations to assess cytology of the cells in the effusion, paraffin-embedded cell blocks for morphology and immunohistochemistry and, where possible, a cell suspension for flow cytometric immunophenotyping.18 On cellular smears and cytospin preparations, the neoplastic cells of BIA-ALCL are relatively easily identified as malignant. They are large, pleomorphic cells with irregular cell membranes, abundant, vacuolated cytoplasm, and large polymorphic, frequently multilobated nuclei and prominent nucleoli (Figure 3A-B). However, cytological features overlap with other malignant conditions, in particular with high-grade breast carcinomas; therefore, adequate immunophenotyping, ideally with immunohistochemistry (Figure 3C) and flow cytometry (Figure 3E-G), is required for a definitive diagnosis.
As discussed in “Surgical Management,” surgical removal of the implant, total capsulectomy, and complete removal of any disease or mass with negative margins remains the main therapy of BIA-ALCL. Thus, careful examination of the capsulectomy specimen and extensive sampling of both the inner part of the capsule adjacent to the implant and the outer part adjacent to the skin or breast are essential to confirm diagnosis and to establish depth of invasion and negative margins. In histological sections, most of the neoplastic cells BIA-ALCL are seen embedded in the fibrinous exudate adjacent to the implant (Figure 2). However, in a subset of the cases, invasion into the fibrous capsule and beyond, and rarely, dissemination to the axillary lymph nodes can be seen.34 The neoplastic cells often form cohesive clusters and have cytological features similar to systemic ALCL35,36 (Figure 3). Immunophenotyping is essential to confirm diagnosis. By definition, the neoplastic cells strongly and uniformly express CD30 with a membranous and Golgi pattern, frequently CD4, but often lack expression of other T-cell–specific markers, such as CD3 and CD5, and also lack expression of ALK.35-37 In addition, CD30 could be expressed by normal lymphocytes, carcinomas, and myeloid lineage cells. Therefore, a comprehensive T-cell phenotyping panel is required to establish a neoplastic phenotype. If BIA-ALCL spreads beyond the implant capsule into adjacent tissues or regional lymph nodes, it cannot be distinguished from systemic ALK-negative ALCL by morphology, immunophenotype, or genetic features alone. Close interaction between surgeons, radiologists, and pathologist is essential for accurate diagnosis and staging. BIA-ALCL is negative for DUSP22 and TP63; however, these are not routine tests in the diagnosis of the disease.38
Case 1 continued
The patient was referred for ultrasound of the bilateral breasts. Ultrasound of the right breast was unremarkable. Ultrasound of the left breast showed a large effusion surrounding her breast implant without evidence of axillary lymphadenopathy. Fine needle aspirate of the seroma showed predominantly lymphocytes that express CD4, CD2, and CD30 and do not express CD3, or CD8 by flow cytometry. Cytology showed no evidence of carcinoma. PET/CT was performed and showed no evidence of lymphadenopathy or suspicion for lymphoma outside of the left breast.
Case 2 continued
The patient underwent a core needle biopsy of the lymph node that showed large atypical lymphocytes highly suspicious for lymphoma. There was no evidence of breast cancer on the biopsy. Flow cytometry sent from the aspiration of the seroma showed large T cells that also expressed CD4 and CD30 but not CD5 or CD8. PET/CT showed a hypermetabolic 2-cm mass on the capsule of the left breast implant as well as 2.5-cm and 3-cm hypermetabolic lymph nodes in the left axilla. There were no areas suspicious for lymphoma elsewhere.
Highly suspected or confirmed BIA-ALCL
Our approach to highly suspected or confirmed cases of BIA-ALCL is summarized in Figure 4. In cases of histologically confirmed BIA-ALCL or high suspicion for BIA-ALCL, patients, if possible, should undergo PET/CT to evaluate for presence of capsular masses, chest wall invasion, and in particular, disease outside of the breast prior to any surgical intervention. We prefer PET/CT prior to surgical therapy in known or highly suspected cases of BIA-ALCL because postoperative inflammation or wound healing may confound the interpretation of the chest wall and ipsilateral axilla. In addition, suspicious regional nodes identified preoperatively may be sampled at the time of surgery. The diagnosis of disseminated disease should be confirmed histologically. Bone marrow biopsies can be reserved for cases of high suspicion for bone marrow involvement or in cases that present with unexplained cytopenias. The FDA specifically recommends all confirmed cases should be reported to the American Society of Plastic Surgeons PROFILE patient registry (www.thepsf.org/PROFILE). The PROFILE registry prospectively tracks disease characteristics and oncologic outcomes of BIA-ALCL as well as supports a centralized tissue bank through collaboration with MD Anderson Cancer Center. As of June 2018, the PROFILE registry has received data on 226 unique cases of BIA-ALCL in the United States and was aware of 561 unique cases worldwide.
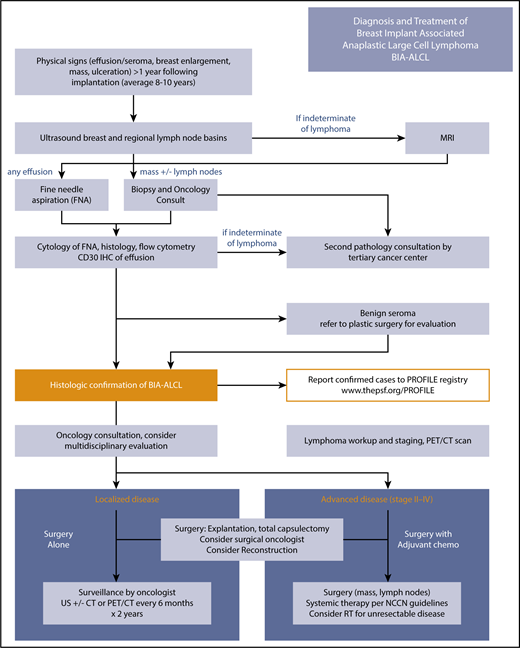
BIA-ALCL treatment algorithm. Diagnosis and treatment follow NCCN guidelines. The essential elements are summarized in the algorithm in the artwork. US, ultrasound.
BIA-ALCL treatment algorithm. Diagnosis and treatment follow NCCN guidelines. The essential elements are summarized in the algorithm in the artwork. US, ultrasound.
Staging and prognosis
Using the Lugano revision of the Ann Arbor Staging System, nearly all BIA-ALCL patients have early-stage disease, either stage 1E (83% to 84%) or stage IIE (10% to 16%).39 A validated BIA-ALCL–specific TNM staging system has been encouraged by the NCCN because the vast majority of patients present with localized disease that can be successfully treated with surgery alone7 (Figures 2C and 4). Patients with stage I disease have lymphoma evidenced in the effusion or penetrating into the capsule without extension beyond the capsule of the implant. Those with stage IIA disease have disease limited to the breast but with evidence of lymphoma invading beyond the capsule into surrounding tissue. Patients with stage IIB disease have involvement of a single regional lymph node. Any disease outside of the ipsilateral breast and regional lymph node basins is considered stage IV disease. Metastases to liver, small bowel, and bone have been observed. To date, 16 disease-related deaths have been reported, most commonly following invasion into the chest wall and mediastinum. In one of the earlier series of BIA-ALCL, patients with stage I disease were found to have a 100% 3-year overall survival and 63% 3-year event-free survival (EFS), as defined by lymphoma recurrence, persistence, progression, or death.40 In a cohort of 87 patients, those with stage I disease had a 93% overall survival and 63% EFS at 3 years.7 This demonstrates that those who have localized disease can be at risk of local recurrence but still have excellent overall survival. Those with stage II disease had 63% 3-year EFS, and those with stage III disease had 29% 3-year EFS, as these patients were at higher risk of local recurrence. In this study, Clemens et al7 demonstrated that those who underwent complete surgical excision for localized BIA-ALCL had improved outcomes, and surgery is a critical component of the management of these patients (Figure 5).
Survival curves according to treatment approaches and TNM tumor staging. (A-B) treatment approaches and (C-D) TNM tumor staging; (A,C) EFS, (B,D) overall survival. CS, complete surgery; LS, limited surgery; XRT, external beam radiation. Reprinted from Clemens et al7 with permission.
Survival curves according to treatment approaches and TNM tumor staging. (A-B) treatment approaches and (C-D) TNM tumor staging; (A,C) EFS, (B,D) overall survival. CS, complete surgery; LS, limited surgery; XRT, external beam radiation. Reprinted from Clemens et al7 with permission.
Surgical management
Timely diagnosis followed by explantation with complete excision of any mass and the surrounding implant capsule is the optimal approach for the management of patients with BIA-ALCL. There is no clear role for radical mastectomy because this is not a disease of breast tissue. Surgery alone is sufficient for disease localized to the capsule and/or a resectable chest wall mass,7,41 representing >80% of patients. Suspicious enlarged lymph node(s) may require biopsy at time of explantation and capsulectomy. Sentinel lymph node biopsy is not feasible based on the sizeable lymph node basin drainage from an implant capsule, and full axillary lymph node dissection does not appear to be efficacious for reducing disease recurrence. As discussed above, presurgery PET/CT however is helpful in identifying nodes suspicious for involvement by lymphoma. Considering ∼4.6% of cases demonstrated bilateral implant capsule involvement, explantation of the contralateral textured breast implant is generally recommended.7 Complete surgical removal of the entire capsule is critically important because retained scar capsule has been associated with disease recurrence and progression. Because of increased local recurrence rates, incomplete resection may require adjuvant therapies such as local radiation therapy or systemic therapy. A preoperative PET/CT scan can guide surgical resection to ensure any associated capsule masses are completely resected. Inadvertent spillage of the effusion during capsulectomy has an unknown significance, but may be unavoidable and has not been observed to influence recurrence rates. Intraoperative frozen section study can guide more precise excision allowing for additional tissue removal if margins return positive; however, pathological assessment is hampered because immediate CD30 immunohistochemistry is not available. We have performed additional surgery for positive postoperative margins status when required. Ideally, the approach to surgical ablation should take into consideration the ultimate aesthetic outcome, such as use of existing breast scars, an inframammary fold incision approach, or explantation in combination with a mastopexy to allow for mound reshaping and skin tailoring. For patients with localized disease that is completely resected, oncologic outcomes have been excellent with the majority of patients remaining disease free long term. Those with local residual disease, positive margins, or unresectable disease with chest wall invasion may benefit from further adjuvant treatment.
With regards to the timing for reconstructive surgery, there are no standard guidelines. Replacement with a textured implant is discouraged. We have employed both immediate reconstruction (for disease limited to the seroma and capsule) and delayed reconstructions at 6 months to 2 years utilizing autologous tissue reconstruction, serial fat grafting, and smooth implant reconstruction. In our collective experience, we have had 1 patient with recurrence at 2 years following reconstruction with a smooth implant. Although that patient was successfully treated with repeat explantation, it is unclear whether this was related to an incomplete resection at her primary surgery or due to the replacement implant. Type and timing of reconstruction are determined based on extent of disease, ability to resect, and patient’s wishes and priorities.
Adjuvant therapy
There is no established standard approach to the treatment of patients who are thought to be at higher risk of local recurrence, such as those with mass with incomplete excision and/or lymph node metastasis. Patients have been treated with adjuvant radiation and/or with adjuvant chemotherapy in this setting.6,7 However, others have successfully been treated with radiation therapy or chemotherapy only after documented local recurrence as well.6,13 At this time, the role of adjuvant therapy with chemotherapy, brentuximab vedotin, or radiation therapy in patients at high risk of recurrence remains unknown. Patients with evidence of disease beyond the capsule have higher risk of recurrence compared with those who have disease limited to the capsule. Therefore, for those who have residual localized disease or if complete excision is not possible, we believe localized radiation (24-36 Gy) following surgery is reasonable.3,42 Patients with a history of prior breast radiation exposure may be considered for adjuvant chemotherapy or brentuximab vedotin. However, this is an uncommon situation, and there is little experience to confirm the clinical benefit with this approach. Those who develop recurrent disease outside of the breast should be considered for systemic treatment.
Case 1 continued
The patient underwent bilateral complete surgical resection of the capsule and implant. Evaluation of the capsule showed no evidence of capsular invasion or masses. The fluid surrounding the implant showed a population of large atypical lymphocytes that expressed CD4, CD2, and CD30, and did not express CD3, or CD8 by flow cytometry. Following complete surgical resection, she has remained on surveillance and without evidence of disease for 4 years.
Case 2 continued
The patient underwent surgical resection of bilateral implants and the capsule with negative margins. There was a 1-cm mass that invaded but did not appear to penetrate through the capsule, which was composed of atypical lymphocytes that were positive for CD3, CD4, and CD30 and negative for CD5, CD7, and CD8 by immunohistochemistry, which was consistent with BIA-ALCL. Biopsy of the lymph node at the time of surgery showed large atypical lymphocytes, which by immunohistochemistry stained positive for CD3, CD4, CD30, and negative for CD5, CD7, and CD8.
Echocardiogram demonstrated an ejection fraction of 65% without evidence of wall motion abnormalities. She underwent 3 cycles of cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone (CHOEP) followed by 3 additional cycles of CEOP (cyclophosphamide, vincristine, etoposide, prednisone). PET/CT following completion of therapy showed a complete metabolic response.
Initial systemic treatment
As the vast majority of patients with BIA-ALCL have had localized disease, there is no standard approach for systemic treatment of these patients.
True advanced disease with BIA-ALCL, as opposed to systemic ALCL in a person with breast implants, is rare and constitutes involvement in lymph nodes or other organs. In the largest series of patients with lymph node involvement (n = 14), the 5-year overall survival was 75% for those with lymph node involvement compared with 97.9% in those without lymph node involvement at presentation.34 Patients with advanced disease have most often been treated with combination chemotherapy: cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP), CHOEP, or other anthracycline-based chemotherapy. Patients who have failed CHOP-based chemotherapy have been reported to have a complete response to brentuximab vedotin.43,43-45 Rare patients have also been treated with ABVD (adriamycin, bleomycin, vinblastine, dacarbazine); hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine); and ICE (ifosfamide, carboplatin, etoposide).7,15,46 By extrapolation and experience, we approach these patients with curative intent as we do for those with systemic ALK− ALCL with combination chemotherapy, most often CHOEP.47 Although in other lymphomas, patients with localized disease may be treated with radiation following chemotherapy, administering radiation following chemotherapy in this population has not been prospectively studied. As prior therapy for breast cancer and radiation fields involving the breast are common in these patients, treatment plans should be individualized. For patients with regional lymph nodes only, we consider adding radiation when possible, and for the rare patients with disseminated disease, we consider consolidating first remissions with high-dose chemotherapy and autologous stem cell transplantation. The ECHELON-2 trial investigating the addition of brentuximab vedotin to cyclophosphamide, doxorubicin, and prednisone will provide important prospective data for the treatment of systemic ALCL, although significant new data on BIA-ALCL are not expected.
Patients who have advanced BIA-ALCL with a history of prior chemotherapy present an additional challenge in the treatment of this disease. Those patients with a history of breast cancer who have not had prior exposure to anthracyclines can be treated with systemic chemotherapy as discussed above. For those with significant anthracycline exposure, modified CHOP-based regimens such as CEOP (cyclophosphamide, etoposide, vincristine, prednisone) can be considered. We have successfully treated patients with a prior history of breast cancer who had received doxorubicin 240 mg/m2, with CHOEP for 2 to 3 cycles and then omitted further doxorubicin for the remainder of the course. Brentuximab vedotin may also be considered for those who are not candidates for anthracycline-based therapy.48 However, the literature and experience regarding this clinical scenario remain scant.
Relapsed disease
Patients with advanced relapsed BIA-ALCL who have been treated with systemic therapy are treated similarly to those with recurrent ALK− ALCL. Those patients who experience systemic relapse after localized therapy can be treated similar to those with newly diagnosed systemic ALCL.
As brentuximab vedotin is FDA approved for relapsed ALCL with an overall response rate of 86%, it is a reasonable treatment option in this population.49 Efforts to evaluate the mutational profile of patients with BIA-ALCL have demonstrated that these tumors have recurrent mutations in JAK1 and STAT3, suggesting that JAK/STAT inhibitors are worthy of future investigation in this disease.30,31
Disease surveillance
NCCN recommends observation with a clinical follow-up, history, and physical examination every 3 to 6 months for 2 years and then as clinically indicated. We often follow with PET/CT scans every 6 months for 2 years and then only as clinically indicated to screen for systemic recurrence. Other radiation minimizing imaging modalities such as MRI or ultrasound can be considered but are less useful for detecting relapse outside the breast.
Conclusion
BIA-ALCL is a rare disease that can develop years after placement of a textured breast implant, and our understanding of the disease is evolving. Periprosthetic effusions >1 year after implantation should be aspirated and screened for lymphoma, including CD30 immunohistochemistry and flow cytometry. Complete surgical excisions including total capsulectomy are sufficient treatment in the majority of cases. The NCCN has established diagnosis and management guidelines to direct clinicians. Identified cases should be reported to the PROFILE registry.
Acknowledgments
Graphic design support for Figure 2A was provided by Brenda Lewis at Vaniam Group. Assistance with the pathology section and images in Figure 3 were provided by Ahmet Dogan (Memorial Sloan Kettering Cancer Center).
This work was supported by the Paul Calabresi K12 program (UL1 TR002345) (N.M.-S.). This research was funded, in part, through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 (S.M.H.).
Authorship
Contribution: N.M.-S., M.W.C., and S.M.H. designed the concept and had final approval of manuscript.
Conflict-of-interest disclosure: N.M.-S. receives research support from Bristol Myers-Squibb, Celgene, Verastem, and Genentech. S.M.H. consults for Celgene, Millennium/Takeda, Kyowa-Hakka-Kirin, Seattle Genetics, Forty-Seven, Mundipharma, Verastem. M.W.C. declares no competing financial interests.
Correspondence: Steven M. Horwitz, Department of Medicine, Lymphoma Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: horwitzs@mskcc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal