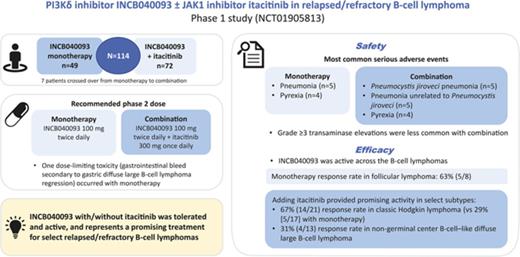
Key Points
INCB040093 was active across lymphoma subtypes, and the addition of itacitinib provided substantial activity in classic Hodgkin lymphoma.
INCB040093 ± itacitinib had a manageable safety and tolerability profile; severe hepatic adverse effects were less common with the combination.
Abstract
Because both phosphatidylinositol 3-kinase δ (PI3Kδ) and Janus kinase (JAK)-signal transducer and activator of transcription pathways contribute to tumor cell proliferation and survival in B-cell malignancies, their simultaneous inhibition may provide synergistic treatment efficacy. This phase 1 dose-escalation/expansion study assessed the safety, efficacy, pharmacokinetics, and pharmacodynamics of INCB040093, a selective PI3Kδ inhibitor, as monotherapy or combined with itacitinib (formerly INCB039110), a selective JAK1 inhibitor, in adult patients with relapsed or refractory (R/R) B-cell lymphomas. Final results are reported. Overall, 114 patients were treated (monotherapy, n = 49; combination therapy, n = 72 [7 patients crossed over from monotherapy to combination]). INCB040093 100 mg twice daily (monotherapy) and INCB040093 100 mg twice daily + itacitinib 300 mg once daily (combination) were the recommended phase 2 doses. One dose-limiting toxicity (gastrointestinal bleed secondary to gastric diffuse large B-cell lymphoma [DLBCL] regression) occurred with monotherapy. The most common serious adverse events with monotherapy were pneumonia (n = 5) and pyrexia (n = 4), and with combination Pneumocystis jiroveci pneumonia (n = 5), pneumonia (unrelated to P jiroveci; n = 5), and pyrexia (n = 4). Grade 3 or higher transaminase elevations were less common with combination. INCB040093 was active across the B-cell lymphomas; 63% of patients (5/8) with follicular lymphoma responded to monotherapy. Adding itacitinib provided promising activity in select subtypes, with responses of 67% (14/21) in classic Hodgkin lymphoma (vs 29% [5/17] with monotherapy) and 31% (4/13) in nongerminal center B-cell-like DLBCL. INCB040093 with/without itacitinib was tolerated and active in this study, and is a promising treatment strategy for patients with select R/R B-cell lymphomas. This trial was registered at www.clinicaltrials.gov as #NCT01905813.
Introduction
Several novel therapeutic strategies are being explored for the treatment of relapsed/refractory (R/R) B-cell lymphomas,1 including agents that target the B-cell receptor signaling network.2 Downstream activation of class I phosphatidylinositol 3-kinases (PI3Ks) by constitutive B-cell receptor signaling is a critical driver of the development of human B-cell lymphomas.3-5 Among the class I PI3K isoforms, phosphatidylinositol 3-kinase δ (PI3Kδ) is a particularly important mediator of B-cell receptor signaling6 ; overexpression and/or constitutive activation of PI3Kδ is associated with B-cell proliferation and survival in B-cell lymphomas.7,8 B-cell receptor-mediated activation of the nuclear factor κB (NF-κB) pathway by PI3K may be synergistically augmented by Janus kinase (JAK)-mediated signaling; interleukins (IL) secreted as a result of NF-κB pathway activation may initiate JAK signal-transducer and activator of transcription (STAT) pathway signaling, resulting in the additional release of cytokines and further JAK-STAT pathway activation by either an autocrine or a paracrine mechanism.9 The JAK-STAT pathway is an attractive therapeutic target for B-cell malignancies. Aberrant STAT3 expression is associated with poor survival in patients with diffuse large B-cell lymphoma (DLBCL) who previously received rituximab.10 Increased STAT3 activity may be associated with constitutive JAK2 activation in some patients with DLBCL,11 and the resulting elevated IL-6 and IL-10 levels are associated with poor patient outcomes.11,12 In addition, recent data have demonstrated that JAK signaling can induce malignant changes through epigenetic modulation, leading to increased proliferation and survival; these changes could be reversed through inhibition of JAK1.13 Because both the PI3Kδ and JAK-STAT pathways contribute to tumor growth and prolong malignant cell survival in B-cell lymphomas, we hypothesized that simultaneously inhibiting both pathways may provide synergistic treatment efficacy. Inhibition of JAK1 may be particularly beneficial considering that among the IL subtypes, IL-6 and IL-10 appear to preferentially signal through the JAK1 isoform.13,14
INCB040093 is an orally administered selective PI3Kδ inhibitor (half maximal inhibitory concentration [IC50] = 31 ± 12 nM), with 74- to more than 900-fold selectivity for PI3Kδ compared with other PI3K isoforms. INCB040093 potently inhibits PI3Kδ-mediated cell growth, induces apoptosis in cell-based assays of human B-cell lymphomas, and inhibits tumor growth in human hematologic xenograft models.15 Importantly, INCB040093-induced apoptosis is inhibited by IL-10-mediated activation of the JAK-STAT pathway in DLBCL cell lines; this inhibitory effect on apoptosis is reversed with JAK1/2 inhibitor treatment.16
Itacitinib (formerly INCB039110) is an oral inhibitor of the JAK family of protein tyrosine kinases with more than 20-fold and more than 100-fold selectivity for JAK1 over JAK2 and JAK3, respectively.17 Doses as high as 600 mg once daily have been tested in clinical trials, with activity reported in patients treated with doses between 200 and 600 mg once daily in indications including myelofibrosis, graft-versus-host disease, rheumatoid arthritis, and psoriasis.17-20 Common toxicities observed in the itacitinib monotherapy studies include fatigue, gastrointestinal toxicities, myelosuppression, and immunosuppression.17-20
Here we report the final results from a phase 1 study of INCB040093 as monotherapy and in combination with itacitinib for the treatment of patients with R/R B-cell lymphomas.
Methods
Study design and patients
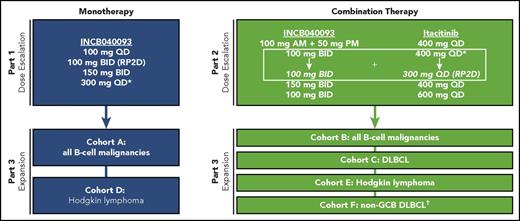
This phase 1 open-label study consisted of 3 parts: INCB040093 monotherapy dose escalation (part 1), INCB040093 + itacitinib combination therapy dose escalation (part 2), and monotherapy and combination therapy dose expansion (part 3, 6 cohorts; NCT01905813; Figure 1).
Study design. *Reduced to INCB040093 100 mg twice daily (BID) and itacitinib 300 mg once daily (QD) in expansion cohorts based on safety data beyond the DLT observation period (represents RP2D for the combination). †As confirmed by immunohistochemistry.40 GCB, germinal center B-cell-like; RP2D, recommended phase 2 dose.
Study design. *Reduced to INCB040093 100 mg twice daily (BID) and itacitinib 300 mg once daily (QD) in expansion cohorts based on safety data beyond the DLT observation period (represents RP2D for the combination). †As confirmed by immunohistochemistry.40 GCB, germinal center B-cell-like; RP2D, recommended phase 2 dose.
Eligible adults had B-cell lymphoid malignancies including Hodgkin lymphoma (HL) and non-Hodgkin B-cell lymphoma (NHL). Patients with Burkitt lymphoma and precursor B-lymphoblastic leukemia/lymphoma were excluded. All patients had life expectancy of at least 12 weeks, had received at least 1 prior treatment regimen, were not eligible for stem cell transplant or other potentially curative therapies, and had an Eastern Cooperative Oncology Group performance status of 2 or lower.
Patients were excluded if they had lymphoma with central nervous system involvement (primary central nervous system lymphoma permitted in expansion cohort B), or had received allogeneic hematopoietic stem cell transplant within 6 months or autologous hematopoietic stem cell transplant within 3 months. Additional exclusion criteria are provided in supplemental Data, available on the Blood website. Patients treated with prior PI3Kδ inhibitors were not excluded from participation in the combination cohort, and patients who discontinued INCB040093 monotherapy treatment were eligible to enroll in the combination cohorts.
The study was conducted in accordance with the study protocol, Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonisation, and applicable regulatory requirements. All patients provided written, informed consent before study participation. The study protocol was approved by the respective institutional review boards or independent ethics committees.
Dosing
In all 3 parts, patients self-administered both INCB040093 (formulated as 50-mg sustained-release oral tablets) and itacitinib (100-mg sustained-release oral tablets). Patients received treatment until unacceptable toxicity, disease progression, or withdrawal of consent.
Part 1 used an accelerated titration design, with initial dose cohorts consisting of a single patient each; dosing started at 100 mg INCB040093 once daily, escalating by up to 2-fold in successive cohorts (either by increasing the number of tablets taken or by increasing dosing frequency) until a grade 2 toxicity was observed, at which time the cohort was expanded to 3 patients. Subsequent cohorts in part 1 were enrolled using a 3 + 3 design, and doses were escalated by no more than 50%. A 21-day observation period was included before each dose escalation. The RP2D was selected to be either the maximum tolerated dose (defined as 1 dose level below that at which at least 33% of patients experienced dose-limiting toxicities [DLTs] during the first 21 days of treatment) or a dose that was tolerated and produced sufficient pharmacologic target inhibition at trough serum levels. A DLT was defined as any grade 3 or higher nonhematologic toxicity, including grade 3 or higher nausea, vomiting, or diarrhea lasting more than 48 hours and not controlled by maximal antiemetic/antidiarrheal therapy, grade 4 neutropenia lasting at least 7 days or febrile neutropenia, grade 4 thrombocytopenia lasting more than 7 days or any grade thrombocytopenia if associated with clinically significant bleeding, grade 4 anemia, or any specific adverse event (AE) that resulted in a dose delay or reduction in more than one-third of patients. In part 2, combination therapy dose escalation proceeded using a 3 + 3 design, starting with an INCB040093 dose 50% lower than the RP2D followed by subsequent dose escalation up to the RP2D determined in part 1. At each dose, INCB040093 was administered in combination with itacitinib at 400 mg once daily; this was followed by 1 dose escalation of itacitinib to 600 mg once daily to determine whether this dose was tolerated. In both parts 1 and 2, patients who had received study drug or drugs for 17 days or more were evaluable for dose-tolerability assessment. In part 3, additional patients were enrolled at the RP2D for monotherapy or chosen combination doses, up to approximately 10 patients in cohort A and 15 patients in each of cohorts B to F. Patients were followed until 28 to 35 days after treatment discontinuation.
Assessments
Patients were assessed on days 1, 8, and 15 of cycle 1 (1 cycle = 21 days) and day 1 of each subsequent treatment cycle (± 3 days); each assessment followed a prespecified schedule.
The primary endpoint was the safety and tolerability of INCB040093 as monotherapy and in combination with itacitinib, assessed by treatment-emergent AEs, physical examinations, vital signs, electrocardiograms, and laboratory assessments. The severity of AEs was assessed using Common Terminology Criteria for Adverse Events version 4.03.
Secondary endpoints were efficacy of INCB040093 as monotherapy and in combination with itacitinib, measured by overall response rate (ORR; the proportion of patients achieving partial [PR] or complete [CR] response) and pharmacokinetics of INCB040093 (monotherapy and combination) and itacitinib (combination). Objective disease status was assessed every 9 weeks on the basis of established and modified response criteria appropriate for the disease subtype,21-24 and included positron emission tomography (PET) to confirm CRs in 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG)–avid disease as appropriate per response criteria.23
Exploratory endpoints included duration of response (DOR; time from first response to the earlier of death or disease progression), progression-free survival (PFS; time from first dose to the earlier of death or disease progression), and pharmacodynamics (including biomarkers for JAK1 inhibition [ie, whole-blood cytokine-induced pSTAT concentration]25 and PI3Kδ inhibition [ie, whole-blood pAKT concentration]15 ).
Statistical methods
A sample size of 135 or fewer patients was estimated based on enrollment of 3 or fewer single-patient cohorts, with 3 to 6 patients per dose level in part 1, 3 to 6 patients per dose level in part 2, and 10 to 15 patients in each of the 6 expansion cohorts in part 3. Details on the statistical properties for part 3 cohorts are provided in the supplemental Data.
Safety, tolerability, and efficacy analyses were performed on the safety population/intent-to-treat population, which included all patients enrolled in the study who received 1 or more dose of INCB040093 or itacitinib. Pharmacokinetic and pharmacodynamic analyses were performed on the pharmacokinetic/pharmacodynamic population, which included all enrolled patients who had available pharmacokinetic/pharmacodynamic data. All statistical analyses were exploratory in nature and were summarized using descriptive statistics.
Results
Patients
Of 114 patients enrolled at the time of this final analysis (data cutoff: 1 December 2016), 49 received INCB040093 monotherapy (parts 1 and 3), and 72 received INCB040093 + itacitinib combination therapy (parts 2 and 3). This includes 7 patients (all with classic HL [cHL]) who discontinued monotherapy and reenrolled into the study and received combination therapy.
INCB040093 monotherapy.
Of the 49 patients treated with INCB040093 monotherapy, 46 (94%) discontinued treatment, most commonly because of disease progression, followed by an AE (supplemental Figure 1). Study treatment was ongoing in 3 patients (6%) at the time of this analysis.
Patients had a median age of 60.0 years and had received a median of 3 prior systemic therapies (Table 1). Overall, 65% patients had NHL (with CLL/SLL as the most common type [24%]), and 35% had HL (with cHL accounting for all cases.
Patient demographics and baseline characteristics (ITT)
| Characteristic . | Monotherapy (n = 49) . | Combination therapy (n = 72) . |
|---|---|---|
| Age, median (range), y | 60.0 (21-89) | 60.5 (22-85) |
| >65 y, n (%) | 21 (43) | 21 (29) |
| Male, n (%) | 38 (78) | 44 (61) |
| Race, n (%) | ||
| White | 43 (88) | 62 (86) |
| African American | 3 (6) | 6 (8) |
| Asian | 2 (4) | 0 |
| Unknown | 1 (2) | 4 (6) |
| ECOG performance status, n (%) | ||
| 0 | 22 (45) | 28 (39) |
| 1 | 25 (51) | 41 (57) |
| 2 | 1 (2) | 2 (3) |
| Missing | 1 (2) | 1 (1) |
| Prior systemic therapies | ||
| Median (range) | 3 (1-12) | 3 (1-13) |
| Number, n (%) | ||
| 1 | 5 (10) | 10 (14) |
| 2 | 11 (22) | 15 (21) |
| 3 | 11 (22) | 14 (19) |
| 4 | 5 (10) | 12 (17) |
| ≥5 | 17 (35) | 21 (29) |
| Prior radiation, n (%) | 18 (37) | 29 (40) |
| Prior hematopoietic stem cell transplant, n (%) | 15 (31) | 21 (29) |
| Median (range) duration since initial diagnosis, years | 4.1 (0.8-18.0) | 3.2 (0.3-24.1) |
| Lymphoma subtype, n (%) | ||
| NHL | 32 (65) | 50 (69) |
| CLL/SLL | 12 (24) | 1 (1) |
| FL | 8 (16) | 11 (15) |
| MCL | 6 (12) | 0 |
| DLBCL | 2 (4) | 23 (32) |
| GCB | 0 | 9 (13) |
| Non-GCB | 0 | 13 (18) |
| Unknown | 2 (4) | 1 (1) |
| PMBCL | 0 | 2 (3) |
| Transformed NHL histology | 1 (2) | 8 (11) |
| MZL | 2 (4) | 3 (4) |
| Splenic | 1 (2) | 2 (3) |
| Nodal | 1 (2) | 1 (1) |
| WM | 1 (2) | 2 (3) |
| HL | 17 (35) | 22 (31) |
| cHL | 17 (35) | 21 (29) |
| Nodular sclerosis HL | 15 (31) | 18 (25) |
| Mixed-cellularity HL | 2 (4) | 2 (3) |
| Subtype unknown | 0 | 1 (1) |
| NLP HL | 0 | 1 (1) |
| Prior therapies in cHL | ||
| Prior systemic therapies | ||
| Median (range) | 4 (1-12) | 4 (1-13) |
| Number, n (%) | ||
| 1 | 2 (12) | 1 (5) |
| 2 | 0 | 3 (14) |
| 3 | 4 (24) | 4 (19) |
| 4 | 3 (18) | 3 (14) |
| ≥5 | 8 (47) | 10 (48) |
| Prior treatment with brentuximab, n (%) | 14 (82) | 15 (71) |
| Prior radiation, n (%) | 9 (53) | 11 (52) |
| Prior hematopoietic stem cell transplant, n (%) | 11 (65) | 14 (67) |
| Characteristic . | Monotherapy (n = 49) . | Combination therapy (n = 72) . |
|---|---|---|
| Age, median (range), y | 60.0 (21-89) | 60.5 (22-85) |
| >65 y, n (%) | 21 (43) | 21 (29) |
| Male, n (%) | 38 (78) | 44 (61) |
| Race, n (%) | ||
| White | 43 (88) | 62 (86) |
| African American | 3 (6) | 6 (8) |
| Asian | 2 (4) | 0 |
| Unknown | 1 (2) | 4 (6) |
| ECOG performance status, n (%) | ||
| 0 | 22 (45) | 28 (39) |
| 1 | 25 (51) | 41 (57) |
| 2 | 1 (2) | 2 (3) |
| Missing | 1 (2) | 1 (1) |
| Prior systemic therapies | ||
| Median (range) | 3 (1-12) | 3 (1-13) |
| Number, n (%) | ||
| 1 | 5 (10) | 10 (14) |
| 2 | 11 (22) | 15 (21) |
| 3 | 11 (22) | 14 (19) |
| 4 | 5 (10) | 12 (17) |
| ≥5 | 17 (35) | 21 (29) |
| Prior radiation, n (%) | 18 (37) | 29 (40) |
| Prior hematopoietic stem cell transplant, n (%) | 15 (31) | 21 (29) |
| Median (range) duration since initial diagnosis, years | 4.1 (0.8-18.0) | 3.2 (0.3-24.1) |
| Lymphoma subtype, n (%) | ||
| NHL | 32 (65) | 50 (69) |
| CLL/SLL | 12 (24) | 1 (1) |
| FL | 8 (16) | 11 (15) |
| MCL | 6 (12) | 0 |
| DLBCL | 2 (4) | 23 (32) |
| GCB | 0 | 9 (13) |
| Non-GCB | 0 | 13 (18) |
| Unknown | 2 (4) | 1 (1) |
| PMBCL | 0 | 2 (3) |
| Transformed NHL histology | 1 (2) | 8 (11) |
| MZL | 2 (4) | 3 (4) |
| Splenic | 1 (2) | 2 (3) |
| Nodal | 1 (2) | 1 (1) |
| WM | 1 (2) | 2 (3) |
| HL | 17 (35) | 22 (31) |
| cHL | 17 (35) | 21 (29) |
| Nodular sclerosis HL | 15 (31) | 18 (25) |
| Mixed-cellularity HL | 2 (4) | 2 (3) |
| Subtype unknown | 0 | 1 (1) |
| NLP HL | 0 | 1 (1) |
| Prior therapies in cHL | ||
| Prior systemic therapies | ||
| Median (range) | 4 (1-12) | 4 (1-13) |
| Number, n (%) | ||
| 1 | 2 (12) | 1 (5) |
| 2 | 0 | 3 (14) |
| 3 | 4 (24) | 4 (19) |
| 4 | 3 (18) | 3 (14) |
| ≥5 | 8 (47) | 10 (48) |
| Prior treatment with brentuximab, n (%) | 14 (82) | 15 (71) |
| Prior radiation, n (%) | 9 (53) | 11 (52) |
| Prior hematopoietic stem cell transplant, n (%) | 11 (65) | 14 (67) |
CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL, follicular lymphoma; ITT, intent-to-treat; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NA, not applicable; NLP HL, nodular lymphocytic-predominant Hodgkin lymphoma; PMBCL, primary mediastinal large B-cell lymphoma; WM, Waldenström macroglobulinemia.
INCB040093 + itacitinib combination.
Of the 72 patients treated with INCB040093 + itacitinib combination, 61 (85%) discontinued treatment; as with monotherapy, discontinuation was most common because of disease progression, followed by an AE (supplemental Figure 1). Study treatment was ongoing in 11 patients (15%) at the time of this analysis.
Patients had a median age of 60.5 years and had received a median of 3 prior systemic therapies (Table 1). Overall, 69% patients had NHL (with DLBCL as the most common type [32%]), and 31% had HL (with cHL accounting for all but 1 case.
Exposure, safety, and tolerability
INCB040093 monotherapy.
The median duration of treatment among the 49 patients was 180 days (range, 5-983 days). In part 1 (dose escalation), 1 DLT was observed with INCB040093 100 mg twice daily (grade 3 gastrointestinal hemorrhage secondary to regression of gastric DLBCL); this DLT resolved upon treatment discontinuation. Although dose escalation to INCB040093 150 mg twice daily was tolerated within the initial DLT observation period, grade 3 or higher increases in alanine aminotransferase (ALT; 27%) and aspartate aminotransferase (AST; 20%) were observed beyond this period. Therefore, all patients ongoing at 150 mg twice daily had the dose reduced to 100 mg twice daily; this reduced dose was selected for further expansion in part 3 and was designated as R2PD. This dose also was found to significantly inhibit pAKT (see below). Among the 28 patients in the INCB040093 100 mg twice daily cohort, the incidence of grade 3 or higher elevations of ALT and AST was similar to that observed in the 150 mg twice daily cohort (21% each).
The most common (≥30%) all-cause nonlaboratory AEs were diarrhea (39%), fatigue (35%), cough (33%), nausea (33%), and headache (31%; Table 2). The most common (>1 patient) nonlaboratory grade 3 or higher AEs were pneumonia unrelated to Pneumocystis jiroveci (n = 5, 10%), abdominal pain, colitis, diarrhea, dyspnea, pleural effusion, and pyrexia (n = 2 each, 4%). One patient experienced grade 3 pneumonitis. Infections and infestation events (MedDRA System Organ Class) occurred in 28 patients (57%); the majority were grade 1/2, with grade 3 events in 8 patients (16%). Twenty-three patients (47%) experienced a serious AE; most common (≥3 patients) were pneumonia unrelated to P jiroveci (n = 5, 10%) and pyrexia (n = 4, 8%). Treatment-emergent laboratory abnormalities of interest (liver enzyme elevations, anemia, neutropenia, and thrombocytopenia) are presented in Table 2. In general, ALT/AST elevations occurred after approximately 6 weeks of study treatment, with a median time to onset of grade 3/4 ALT elevations of 42.5 (range, 22-106) days, and of grade 3/4 AST elevations of 43 (range, 30-106) days. Most ALT/AST-increased AEs were reversible with treatment interruption and/or dose reduction (supplemental Data); however, 3 patients discontinued treatment because of AEs of ALT elevations.
Nonlaboratory AEs and treatment-emergent laboratory abnormalities of interest occurring in patients receiving PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib (safety population)
| Event or abnormality . | Monotherapy (n = 49) . | Combination therapy (n = 72) . | ||
|---|---|---|---|---|
| All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | |
| Nonlaboratory AEs (MedDRA preferred term)* | ||||
| Diarrhea | 19 (39) | 2 (4) | 22 (31) | 1 (1) |
| Fatigue | 17 (35) | 0 | 28 (39) | 1 (1) |
| Cough | 16 (33) | 0 | 31 (43) | 0 |
| Nausea | 16 (33) | 0 | 33 (46) | 2 (3) |
| Headache | 15 (31) | 0 | 14 (19) | 0 |
| Back pain | 14 (29) | 1 (2) | 16 (22) | 1 (1) |
| Decreased appetite | 13 (27) | 0 | 19 (26) | 0 |
| Dyspnea | 13 (27) | 2 (4) | 13 (18) | 0 |
| Pyrexia | 13 (27) | 2 (4) | 26 (36) | 1 (1) |
| Vomiting | 13 (27) | 1 (2) | 25 (35) | 2 (3) |
| Upper respiratory tract infection | 12 (24) | 0 | 14 (19) | 0 |
| Constipation | 11 (22) | 0 | 16 (22) | 1 (1) |
| Chills | 7 (14) | 0 | 23 (32) | 0 |
| Night sweats | 8 (16) | 0 | 19 (26) | 0 |
| Hematologic laboratory abnormalities | ||||
| Neutropenia | 22 (45) | 9 (18) | 35 (49) | 17 (24) |
| Anemia | 15 (31) | 5 (10) | 36 (50) | 7 (10) |
| Thrombocytopenia | 15 (31) | 3 (6) | 50 (69) | 8 (11) |
| Chemical laboratory abnormalities | ||||
| ALT increased | 23 (47) | 10 (20) | 30 (42) | 2 (3) |
| AST increased | 21 (43) | 9 (18) | 41 (57) | 2 (3) |
| Event or abnormality . | Monotherapy (n = 49) . | Combination therapy (n = 72) . | ||
|---|---|---|---|---|
| All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | |
| Nonlaboratory AEs (MedDRA preferred term)* | ||||
| Diarrhea | 19 (39) | 2 (4) | 22 (31) | 1 (1) |
| Fatigue | 17 (35) | 0 | 28 (39) | 1 (1) |
| Cough | 16 (33) | 0 | 31 (43) | 0 |
| Nausea | 16 (33) | 0 | 33 (46) | 2 (3) |
| Headache | 15 (31) | 0 | 14 (19) | 0 |
| Back pain | 14 (29) | 1 (2) | 16 (22) | 1 (1) |
| Decreased appetite | 13 (27) | 0 | 19 (26) | 0 |
| Dyspnea | 13 (27) | 2 (4) | 13 (18) | 0 |
| Pyrexia | 13 (27) | 2 (4) | 26 (36) | 1 (1) |
| Vomiting | 13 (27) | 1 (2) | 25 (35) | 2 (3) |
| Upper respiratory tract infection | 12 (24) | 0 | 14 (19) | 0 |
| Constipation | 11 (22) | 0 | 16 (22) | 1 (1) |
| Chills | 7 (14) | 0 | 23 (32) | 0 |
| Night sweats | 8 (16) | 0 | 19 (26) | 0 |
| Hematologic laboratory abnormalities | ||||
| Neutropenia | 22 (45) | 9 (18) | 35 (49) | 17 (24) |
| Anemia | 15 (31) | 5 (10) | 36 (50) | 7 (10) |
| Thrombocytopenia | 15 (31) | 3 (6) | 50 (69) | 8 (11) |
| Chemical laboratory abnormalities | ||||
| ALT increased | 23 (47) | 10 (20) | 30 (42) | 2 (3) |
| AST increased | 21 (43) | 9 (18) | 41 (57) | 2 (3) |
Nonlaboratory AEs and laboratory abnormalities are discussed separately.
MedDRA, Medical Dictionary for Regulatory Activities.
Nonlaboratory AEs occurring in ≥20% of patients in any treatment group.
AEs led to permanent discontinuation of INCB040093 monotherapy in 11 patients (22%), dose interruption in 27 patients (55%), and dose reduction in 5 patients (10%). Two patients (4%) died because of an AE (cardiorespiratory failure resulting from progression and paraneoplastic pemphigus); both were considered unrelated to treatment.
INCB040093 + itacitinib combination.
Among the 72 patients across all doses, the median duration of INCB040093 + itacitinib combination treatment was 140.5 days (range, 5-953 days). No DLTs were observed for any dose combination tested in part 2 (combination dose escalation). Itacitinib 400 mg once daily was initially selected for expansion as a result of cytopenia-related dose interruptions and reductions within the first 2 cycles of treatment with itacitinib 600 mg once daily. However, as the 400-mg dose caused higher than expected JAK-STAT pathway inhibition, as well as reports of Pneumocystis pneumonia, the dose was subsequently reduced to 300 mg once daily and all patients receiving combination therapy were required to receive a standard prophylaxis regimen for Pneumocystis pneumonia. Therefore, the RP2D for the INCB040093 + itacitinib combination was INCB040093 100 mg twice daily and itacitinib 300 mg once daily.
The most common (≥30%) all-cause nonlaboratory AEs were nausea (46%), cough (43%), fatigue (39%), pyrexia (36%), vomiting (35%), chills (32%), and diarrhea (31%; Table 2). Among the 56 patients (78%) with grade 3 or higher nonlaboratory AEs, the most frequent (≥3 patients) were hypoxia and P jiroveci pneumonia (n = 5 each, 7%), pneumonia (unrelated to P jiroveci) and respiratory failure (n = 4 each, 6%), and herpes zoster and urinary tract infection (n = 3 each, 4%). Two patients (3%) had grade 3 colitis and 2 (3%) had grade 3 pneumonitis. Infections and infestations (MedDRA System Organ Class) occurred in 50 patients (69%); the majority were grade 1/2, with grade 3 events occurring in 13 patients (18%) and grade 4 in 3 patients (4%); 3 patients (4%) discontinued INCB040093, and 4 patients (6%) discontinued itacitinib because of these AEs. Forty patients (56%) experienced serious AEs, most commonly (≥3 patients) P jiroveci pneumonia (n = 5, 7%), pneumonia (n = 5, 7%), pyrexia (n = 4, 6%), urinary tract infection (n = 3, 4%), and hypoxia (n = 3, 4%).
Treatment-emergent laboratory abnormalities of interest are presented in Table 2. All-grade neutropenia, anemia, and thrombocytopenia occurred in 35 (49%), 36 (50%), and 50 (69%) patients, respectively, with grade 3 or higher events in 17 (24%), 7 (10%), and 8 (11%) patients, respectively. The incidence of grade 3 or higher ALT and AST elevations with combination therapy was notably lower than with monotherapy (3% vs 20% and 3% vs 18%, respectively). Of the 2 patients who had discontinued monotherapy because of ALT/AST elevations and were rechallenged with combination therapy, 1 was able to be carefully titrated up the recommended dose of the combination and continued on study at the time of data cutoff.
Among patients receiving combination treatment, AEs led to permanent discontinuation of INCB040093 in 14 (19%) and itacitinib in 15 (21%) patients, dose interruption of INCB040093 in 42 (58%) and itacitinib in 40 (56%) patients, and dose reduction of INCB040093 in 1 (1%) and itacitinib in 5 (7%) patients. Nine patients (12.5%) died because of an AE (hypoxia and cardiac arrest [n = 2 each], small bowel obstruction, pneumonia, P jiroveci pneumonia, disease progression, and death of an unknown cause [n = 1 each]). Two of these fatal AEs were deemed related to treatment (pneumonia [n = 1] and P jiroveci pneumonia [n = 1]); whether the 1 fatal AE of unknown cause was treatment-related was unknown (the exact date of death also was unknown).
Pharmacokinetic assessments
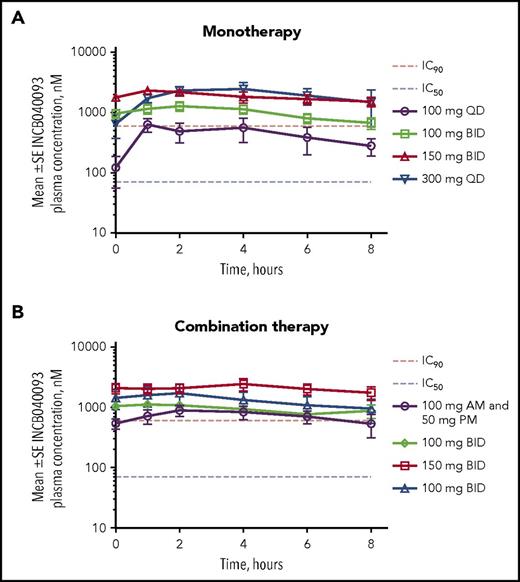
INCB040093 exposures increased linearly with a dose from 100 mg twice daily to 150 mg twice daily. Preliminary mean steady-state plasma concentrations after administration of INCB040093 100 mg twice daily or 150 mg twice daily in combination with itacitinib were similar to those observed with the same doses of INCB040093 monotherapy (Figure 2). The mean trough INCB040093 concentrations for 150 mg twice daily were more than double the in vitro protein-binding adjusted IC90 (700 nM). Tim to maximum INCB040093 plasma concentration is provided in the supplemental Data.
Steady-state plasma concentrations of the PI3Kδ inhibitor INCB040093. (A) Patients receiving multiple doses of INCB040093 monotherapy in Part 1. (B) Patients receiving multiple doses of INCB040093 combined with the JAK1 inhibitor itacitinib in Part 2. IC90, 90% inhibitory concentration; SE, standard error.
Steady-state plasma concentrations of the PI3Kδ inhibitor INCB040093. (A) Patients receiving multiple doses of INCB040093 monotherapy in Part 1. (B) Patients receiving multiple doses of INCB040093 combined with the JAK1 inhibitor itacitinib in Part 2. IC90, 90% inhibitory concentration; SE, standard error.
Pharmacodynamic assessments
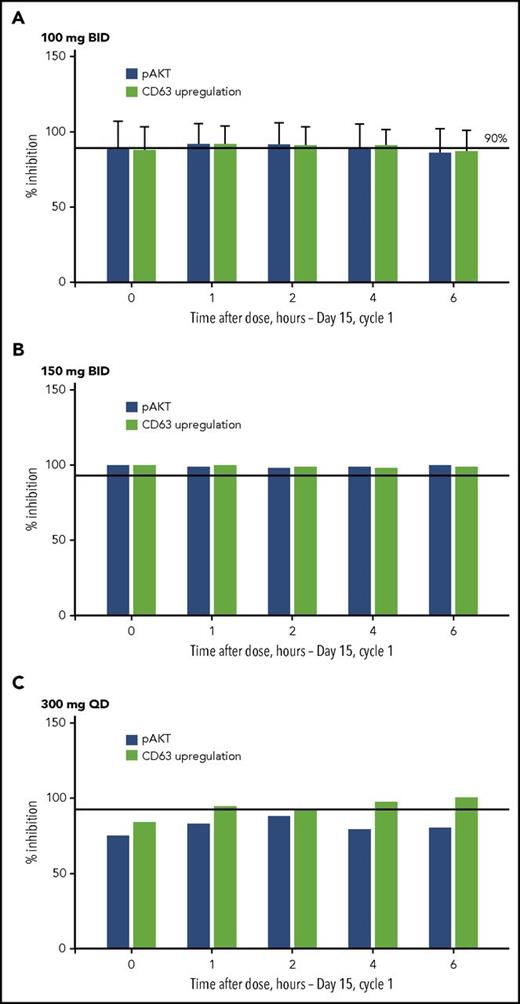
Near-complete PI3Kδ pathway inhibition was achieved with INCB040093 100 mg twice daily; approximately 90% phospho-AKT (downstream effector kinase of PI3K) inhibition was observed at the trough concentration (Figure 3). Similar inhibition also was seen using Fc receptor–mediated upregulation of CD63 in basophils as a marker (Figure 3). For patients treated with INCB040093 in combination with itacitinib 400 mg once daily, IL-6-induced pSTAT3 was decreased by an average of 65% (range, 42%-84%). On the basis of pharmacokinetic exposure data, treatment with INCB040093 in combination with itacitinib 300 mg once daily is estimated to inhibit IL-6 induction of pSTAT3 by an average of 59% (range, 36%-80%).
PI3Kδ signaling inhibition after treatment with the PI3Kδ inhibitor INCB040093. PI3Kδ and JAK1 inhibition as measured using an in vitro assay from whole blood samples (mononuclear cells) obtained on day 15 of cycle 1 from patients receiving INCB040093 at doses of (A) 100 mg BID, (B) 150 mg BID, and (C) 300 mg QD. No target inhibition was observed predose.
PI3Kδ signaling inhibition after treatment with the PI3Kδ inhibitor INCB040093. PI3Kδ and JAK1 inhibition as measured using an in vitro assay from whole blood samples (mononuclear cells) obtained on day 15 of cycle 1 from patients receiving INCB040093 at doses of (A) 100 mg BID, (B) 150 mg BID, and (C) 300 mg QD. No target inhibition was observed predose.
Efficacy
INCB040093 monotherapy.
ORRs are shown in Table 3. For patients with FL, CLL/SLL, MCL, and cHL, the ORR was 63% (5/8; 13% [1/8] CR), 50% (6/12; 0 CRs), 33% (2/6; 17% [1/6] CR), and 29% (5/17; 12% [2/17] CR), respectively. Among patients with other lymphoma types (DLBCL, WM, nodal MZL, splenic MZL, and transformed NHL), the ORR was 50% (3/6; 0 CRs).
Best overall response to PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib, by lymphoma subtype (ITT)
| . | Monotherapy (n = 49) . | Combination therapy (n = 72) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Response category . | cHL (n = 17) . | CLL/SLL (n = 12) . | FL (n = 8) . | MCL (n = 6) . | Other* (n = 6) . | DLBCL (n = 23) . | cHL (n = 21) . | FL (n = 11) . | Transformed NHL (n = 8) . | Other† (n = 9) . |
| ORR, n (%) (95% CI) | 5 (29) (10-56) | 6 (50) (21-79) | 5 (63) (24-91) | 2 (33) (4-78) | 3 (50) (12-88) | 6 (26) (10-48) | 14 (67) (43-85) | 4 (36) (11-69) | 1 (13) (0-53) | 3 (33) (7-70) |
| CR, n (%) | 2 (12) | 0 | 1 (13) | 1 (17) | 0 | 4 (17) | 8 (38) | 1 (9) | 1 (13) | 0 |
| PR, n (%) | 3 (18) | 6 (50) | 4 (50) | 1 (17) | 3 (50) | 2 (9) | 6 (29) | 3 (27) | 0 | 3 (33) |
| SD, n (%) | 6 (35) | 4 (33) | 2 (25) | 3 (50) | 1 (17) | 3 (13) | 2 (10) | 3 (27) | 0 | 4 (44)‡ |
| PD,¶ n (%) | 6 (35) | 0 | 0 | 1 (17) | 1 (17) | 10 (43) | 4 (19) | 2 (18) | 6 (75) | 1 (11) |
| Not assessed/not evaluable,§ n (%) | 0 | 2 (17) | 1 (13) | 0 | 1 (17) | 4 (17) | 1 (5) | 2 (18) | 1 (13) | 1 (11) |
| . | Monotherapy (n = 49) . | Combination therapy (n = 72) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Response category . | cHL (n = 17) . | CLL/SLL (n = 12) . | FL (n = 8) . | MCL (n = 6) . | Other* (n = 6) . | DLBCL (n = 23) . | cHL (n = 21) . | FL (n = 11) . | Transformed NHL (n = 8) . | Other† (n = 9) . |
| ORR, n (%) (95% CI) | 5 (29) (10-56) | 6 (50) (21-79) | 5 (63) (24-91) | 2 (33) (4-78) | 3 (50) (12-88) | 6 (26) (10-48) | 14 (67) (43-85) | 4 (36) (11-69) | 1 (13) (0-53) | 3 (33) (7-70) |
| CR, n (%) | 2 (12) | 0 | 1 (13) | 1 (17) | 0 | 4 (17) | 8 (38) | 1 (9) | 1 (13) | 0 |
| PR, n (%) | 3 (18) | 6 (50) | 4 (50) | 1 (17) | 3 (50) | 2 (9) | 6 (29) | 3 (27) | 0 | 3 (33) |
| SD, n (%) | 6 (35) | 4 (33) | 2 (25) | 3 (50) | 1 (17) | 3 (13) | 2 (10) | 3 (27) | 0 | 4 (44)‡ |
| PD,¶ n (%) | 6 (35) | 0 | 0 | 1 (17) | 1 (17) | 10 (43) | 4 (19) | 2 (18) | 6 (75) | 1 (11) |
| Not assessed/not evaluable,§ n (%) | 0 | 2 (17) | 1 (13) | 0 | 1 (17) | 4 (17) | 1 (5) | 2 (18) | 1 (13) | 1 (11) |
CLL assessed according to Hallek et al21 and Cheson et al22 ; WM assessed according to Owen et al24 ; all other lymphoma subtypes assessed according to Cheson et al.23
PD, progressive disease; SD, stable disease.
DLBCL (n = 2), WM, nodal MZL, splenic MZL, and transformed NHL (n = 1 each).
WM, PMBCL, splenic MZL (n = 2 each), nodal MZL, CLL/SLL, and NLP HL (n = 1 each).
Four patients had SD, including 1 patient with WM who had a minor response, which was not considered a response per protocol.
PD includes relapsed disease (after CR) or PD.
Includes patients without a follow-up response assessment or response assessment that was not evaluable.
Reductions from baseline in target lesion size are shown in Figure 4A, and median DOR data are presented in Table 4. DOR longer than 2 years occurred in a patient with FL (29.3 months; CR ongoing at data cutoff) and in a patient with WM (28.8 months, PR ongoing at data cutoff; supplemental Figure 2).
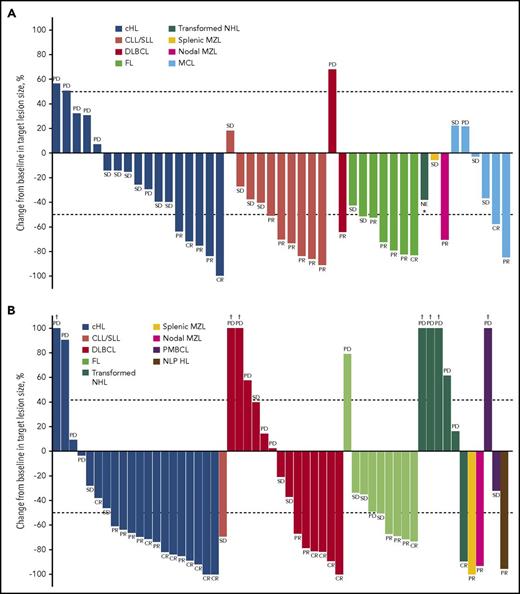
Best percentage change from baseline in target lesion size. (A) Patients receiving the PI3Kδ inhibitor INCB040093 alone. (B) Patients receiving INCB040093 combined with the JAK1 inhibitor itacitinib. Included are patients with data available for best percentage change from baseline in target lesion size (monotherapy, n = 45; combination therapy, n = 55). The upper dotted line corresponds to criteria for PD (at least 50% increase) and lower to PR (at least 50% decrease). PD indicates PD or relapsed disease (after CR). Patients with 18F-FDG–avid lymphoma who were PET-positive at baseline could achieve a CR without complete resolution of the target lesion if the lesion became PET-negative per the Revised Response Criteria for Malignant Lymphoma.23 *Patient was not evaluable for response; the target lesion size was from an assessment, which was 7 days after starting treatment; no other on-study assessment was available. †Best percentage change from baseline in target lesion size >100%.
Best percentage change from baseline in target lesion size. (A) Patients receiving the PI3Kδ inhibitor INCB040093 alone. (B) Patients receiving INCB040093 combined with the JAK1 inhibitor itacitinib. Included are patients with data available for best percentage change from baseline in target lesion size (monotherapy, n = 45; combination therapy, n = 55). The upper dotted line corresponds to criteria for PD (at least 50% increase) and lower to PR (at least 50% decrease). PD indicates PD or relapsed disease (after CR). Patients with 18F-FDG–avid lymphoma who were PET-positive at baseline could achieve a CR without complete resolution of the target lesion if the lesion became PET-negative per the Revised Response Criteria for Malignant Lymphoma.23 *Patient was not evaluable for response; the target lesion size was from an assessment, which was 7 days after starting treatment; no other on-study assessment was available. †Best percentage change from baseline in target lesion size >100%.
Duration of response with PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib, by lymphoma subtype (ITT)
| Tumor subtype . | Median DOR (95% CI), mo . | |
|---|---|---|
| Monotherapy (n = 21) . | Combination therapy (n = 28) . | |
| CLL/SLL | NR (13.9-NE) (n = 6) | — |
| WM | NR (NE-NE) (n = 1) | — |
| MCL | 9.7 (2.1-1 = 7.3) (n = 2) | — |
| FL | 7.6 (0.1-NE) (n = 5) | 11.3 (4.7-NE) (n = 4) |
| Nodal MZL | NR (NE-NE) (n = 1) | NR (NE-NE) (n = 1) |
| Splenic MZL | — | 6.1 (NE-NE) (n = 1) |
| DLBCL | NR (NE-NE) (n = 1) | NR (2.6-NE) (n = 6) |
| Transformed NHL | — | NR (NE-NE) (n = 1) |
| cHL | 8.9 (4.3-NE) (n = 5) | NR (2.6-NE) (n = 14) |
| PMBCL | — | — |
| NLP HL | — | 3.8 (NE-NE) (n = 1) |
| Tumor subtype . | Median DOR (95% CI), mo . | |
|---|---|---|
| Monotherapy (n = 21) . | Combination therapy (n = 28) . | |
| CLL/SLL | NR (13.9-NE) (n = 6) | — |
| WM | NR (NE-NE) (n = 1) | — |
| MCL | 9.7 (2.1-1 = 7.3) (n = 2) | — |
| FL | 7.6 (0.1-NE) (n = 5) | 11.3 (4.7-NE) (n = 4) |
| Nodal MZL | NR (NE-NE) (n = 1) | NR (NE-NE) (n = 1) |
| Splenic MZL | — | 6.1 (NE-NE) (n = 1) |
| DLBCL | NR (NE-NE) (n = 1) | NR (2.6-NE) (n = 6) |
| Transformed NHL | — | NR (NE-NE) (n = 1) |
| cHL | 8.9 (4.3-NE) (n = 5) | NR (2.6-NE) (n = 14) |
| PMBCL | — | — |
| NLP HL | — | 3.8 (NE-NE) (n = 1) |
NE, not estimable; NR, not reached.
INCB040093 + itacitinib combination.
ORRs are shown in Table 3. The highest ORR occurred in patients with cHL (67% [14/21]; 38% [8/21] CR), followed by FL (36% [4/11]; 9% [1/11] CR), DLBCL (26% [6/23]; 17% [4/23] CR), and transformed NHL (13% [1/8]; 13% [1/8] CR). Among patients with other lymphoma types (WM, PMBCL, splenic MZL, nodal MZL, CLL/SLL, and NLP HL), the ORR was 33% (3/9; 0 CRs). Of the 7 patients who had previously received INCB040093 monotherapy (all with cHL), 71% (5/7) responded with combination. Three of the responders (1 with CR and 2 with PR) had previously progressed on monotherapy, and 1 (with CR) had previously achieved stable disease as best response with monotherapy (details on responses among patients who received another PI3Kδ inhibitor before the study entry are included in the supplemental Data). Reductions from baseline in target lesion size for all patients with cHL (monotherapy and combination) are shown in Figure 5.
Best percentage change from baseline in target lesion size in patients with cHL receiving therapy with PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib. Included are patients with data available for best percentage change from baseline in target lesion size (n = 37 [monotherapy, n = 17; combination therapy, n = 20]). The upper dotted line corresponds to criteria for PD (at least 50% increase) and lower to PR (at least 50% decrease). PD indicates PD or relapsed disease (after CR). Patients with 18F-FDG-avid lymphoma who were PET-positive at baseline could achieve a CR without complete resolution of the target lesion if the lesion became PET-negative per the Revised Response Criteria for Malignant Lymphoma.23 *Best percentage change from baseline in target lesion size >100%. †Patients who discontinued INCB040093 monotherapy and re-enrolled into the study to receive combination therapy. ‡Patients who had previously received INCB040093 monotherapy.
Best percentage change from baseline in target lesion size in patients with cHL receiving therapy with PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib. Included are patients with data available for best percentage change from baseline in target lesion size (n = 37 [monotherapy, n = 17; combination therapy, n = 20]). The upper dotted line corresponds to criteria for PD (at least 50% increase) and lower to PR (at least 50% decrease). PD indicates PD or relapsed disease (after CR). Patients with 18F-FDG-avid lymphoma who were PET-positive at baseline could achieve a CR without complete resolution of the target lesion if the lesion became PET-negative per the Revised Response Criteria for Malignant Lymphoma.23 *Best percentage change from baseline in target lesion size >100%. †Patients who discontinued INCB040093 monotherapy and re-enrolled into the study to receive combination therapy. ‡Patients who had previously received INCB040093 monotherapy.
DLBCL also was considered an indication of interest for the combination therapy. As the first 2 patients with nongerminal center B-cell-like (non-GCB) DLBCL had a CR at the first assessment, the protocol was amended to include a cohort of patients with non-GCB DLBCL to further explore the activity of the combination in this population. Overall, the ORR for patients with the non-GCB subtype receiving combination therapy was 31% (4/13; CR, 23% [3/13]); responses (PR and CR) were ongoing at the data cutoff in 2/4 patients (DOR of 9.7 and 8.8 months, respectively).
Reductions from baseline in target lesion size are shown in Figure 4B, and median DOR data are presented in Table 4. DOR longer than 2 years occurred in 2 patients with cHL (25.8 and 25.6 months; both responses were CRs and 1 was ongoing at data cutoff; supplemental Figure 3). In cHL, the median DOR was not yet reached (vs 8.9 months with monotherapy; Table 4). In the 5 patients with cHL who responded to combination therapy after withdrawing from INCB040093 monotherapy, median DOR was 19.4 months. For patients receiving combination vs monotherapy, the median PFS was 6.7 (95% CI, 2.4-NE) vs 5.7 (1.7-NE) months in those with FL, and 23.1 (95% CI, 4.0-NE) vs 8.3 (95% CI, 2.1-11.6) months for those with cHL (Table 5; supplemental Figure 4).
Progression-free survival with PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib, by lymphoma subtype (ITT)
| Tumor subtype . | Monotherapy (n = 49) . | Combination therapy (n = 72) . | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients in disease subgroup, n . | Events, n . | Median PFS (95% CI), mo . | PFS rate at 12 mo (95% CI), % . | No. of patients in disease subgroup, n . | Events, N . | Median PFS (95% CI), mo . | PFS rate at 12 mo (95% CI), % . | |
| NHL | ||||||||
| Indolent | ||||||||
| FL | 8 | 5 | 5.7 (1.7-NE) | 29 (4-61) | 11 | 7 | 6.7 (2.4-NE) | 35 (9-64) |
| WM | 1 | 0 | NR (NE-NE) | 100 (NE-NE) | 2 | 0 | NR (NE-NE) | 100 (NE-NE) |
| Nodal MZL | 1 | 0 | NR (NE-NE) | NR (NE-NE) | 1 | 0 | NR (NE-NE) | 100 (NE-NE) |
| Splenic MZL | 1 | 0 | NR (NE-NE) | NR (NE-NE) | 2 | 2 | 4.8 (0.7-8.9) | 0 (NE-NE) |
| Aggressive | ||||||||
| DLBCL | 2 | 1 | NR (2.1-NE) | 50 (1-91) | 23 | 13 | 2.2 (0.7-6.6) | NE (NE-NE) |
| MCL | 6 | 4 | 12.5 (1.6-20.1) | 50 (11-80) | 0 | — | — | — |
| Transformed NHL | 1 | 0 | NR (NE-NE) | NR (NE-NE) | 8 | 7 | 1.5 (0.1-2.6) | NE (NE-NE) |
| CLL/SLL | 12 | 6 | 16.7 (3.9-NE) | 55 (19-81) | 1 | 1 | 5.4 (NE-NE) | 0 (NE-NE) |
| cHL | 17 | 12 | 8.3 (2.1-11.6) | 8 (1-31) | 21 | 10 | 23.1 (4.0-NE) | 51 (27-71) |
| NLP HL | 0 | — | — | — | 1 | 1 | 8.5 (NE-NE) | 0 (NE-NE) |
| PMBCL | 0 | — | — | — | 2 | 2 | 2.1 (0.7-3.4) | 0 (NE-NE) |
| Tumor subtype . | Monotherapy (n = 49) . | Combination therapy (n = 72) . | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients in disease subgroup, n . | Events, n . | Median PFS (95% CI), mo . | PFS rate at 12 mo (95% CI), % . | No. of patients in disease subgroup, n . | Events, N . | Median PFS (95% CI), mo . | PFS rate at 12 mo (95% CI), % . | |
| NHL | ||||||||
| Indolent | ||||||||
| FL | 8 | 5 | 5.7 (1.7-NE) | 29 (4-61) | 11 | 7 | 6.7 (2.4-NE) | 35 (9-64) |
| WM | 1 | 0 | NR (NE-NE) | 100 (NE-NE) | 2 | 0 | NR (NE-NE) | 100 (NE-NE) |
| Nodal MZL | 1 | 0 | NR (NE-NE) | NR (NE-NE) | 1 | 0 | NR (NE-NE) | 100 (NE-NE) |
| Splenic MZL | 1 | 0 | NR (NE-NE) | NR (NE-NE) | 2 | 2 | 4.8 (0.7-8.9) | 0 (NE-NE) |
| Aggressive | ||||||||
| DLBCL | 2 | 1 | NR (2.1-NE) | 50 (1-91) | 23 | 13 | 2.2 (0.7-6.6) | NE (NE-NE) |
| MCL | 6 | 4 | 12.5 (1.6-20.1) | 50 (11-80) | 0 | — | — | — |
| Transformed NHL | 1 | 0 | NR (NE-NE) | NR (NE-NE) | 8 | 7 | 1.5 (0.1-2.6) | NE (NE-NE) |
| CLL/SLL | 12 | 6 | 16.7 (3.9-NE) | 55 (19-81) | 1 | 1 | 5.4 (NE-NE) | 0 (NE-NE) |
| cHL | 17 | 12 | 8.3 (2.1-11.6) | 8 (1-31) | 21 | 10 | 23.1 (4.0-NE) | 51 (27-71) |
| NLP HL | 0 | — | — | — | 1 | 1 | 8.5 (NE-NE) | 0 (NE-NE) |
| PMBCL | 0 | — | — | — | 2 | 2 | 2.1 (0.7-3.4) | 0 (NE-NE) |
Discussion
The results demonstrate that the PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib at the RP2D is associated with a manageable safety profile and notable preliminary clinical activity in patients with B-cell lymphomas, particularly in cHL. Treatment-emergent laboratory abnormalities of interest and AEs relevant to dose selection were liver enzyme elevations for INCB040093, and cytopenias and infections for itacitinib. Significant inhibition of the PI3K and JAK-STAT pathways was observed, and there was no evidence of a pharmacokinetic interaction between INCB040093 and itacitinib.
AEs after treatment with INCB040093 monotherapy observed here are similar to those observed after single-agent administration of the PI3Kδ inhibitor, idelalisib, in patients with relapsed NHL, including liver enzyme elevations.26 However, distinct safety profiles were observed with the PI3Kδ inhibitor INCB040093 alone and in combination with the JAK1 inhibitor itacitinib. Cough, nausea, chills, and night sweats were experienced by at least 10% of patients receiving combination therapy than monotherapy; however, most of these AEs were grade 1/2 severity. Higher rates of thrombocytopenia (69% with combination vs 31% with monotherapy) and anemia (50% vs 31%) were to be expected, considering these events commonly occur with JAK inhibitors.27 Again, these differences were largely a result of grade 1/2 events. Notably, the incidence of grade 3 or higher ALT and AST elevations was lower with combination therapy (3% each) than with monotherapy (20% and 18%, respectively). Of the 2 patients who experienced grade 3 or higher ALT/AST elevations with INCB040093 monotherapy and crossed over to receive INCB040093 + itacitinib, 1 tolerated the combination without significant liver toxicity after INCB040093 was held initially for 12 days because of grade 2 ALT elevation; INCB040093 was then administered at a total daily dose of 150 mg for 43 days and then escalated to 100 mg twice daily. This patient continued to receive treatment and was in complete remission at 15.4 months of follow-up as of the data cutoff date. However, grade 3 ALT elevation experienced by the other patient was not amenable to dose interruption of both study drugs, and the patient discontinued combination therapy after being treated for 5 days. Nevertheless, these results suggest a potential hepatoprotective effect of itacitinib in the setting of this study, which might involve a blockade of cytokine signaling by JAK1 inhibition, resulting in a concomitant mitigation of cytokine-induced liver inflammation. Consistent with this, preclinical experiments have shown that treatment with ruxolitinib, a JAK1/2 inhibitor, prevented liver toxicity in mice treated with known hepatotoxic agents.28,29 Although the possibility that JAK inhibition exerts a hepatoprotective effect is interesting, this finding may instead reflect divergent populations or other factors related to prior treatment or histology.
The rate of treatment discontinuations resulting from AEs was similar for monotherapy (22%) and combination therapy (19%), suggesting no additional tolerability issues with the addition of a JAK1 inhibitor to PI3Kδ inhibitor therapy. Most of the 11 deaths resulting from AEs (8/11 [73%]) were deemed unrelated to treatment. The 2 treatment-related deaths resulting from P jiroveci pneumonia and pneumonia occurred with INCB040093 100 mg twice daily + itacitinib 400 mg once daily, which represented a higher itacitinib dose than finally selected (300 mg once daily). A higher rate of infections was observed with the combination therapy (69% vs 57% with monotherapy), but the majority were grade 1/2 with both treatment regimens. Taken together, these results suggest that antimicrobial prophylaxis is required with the combination, most notably against P jiroveci pneumonia.
Although comparisons across studies are rendered difficult by differences in study design, including sample sizes, the efficacy of INCB040093 monotherapy is similar to that reported for other PI3Kδ inhibitors. ORR was 63% in FL, 50% in CLL/SLL, and 29% in cHL; these response rates were comparable to those demonstrated with single-agent idelalisib30-32 and duvelisib.33 In the current study, the addition of itacitinib to INCB040093 in cHL appeared to increase treatment activity, approximately doubling the ORR (67% vs 29% with monotherapy, albeit with an overlap in 95% CIs), and prolonged DOR (not yet reached vs 8.9 months) and PFS (23.1 vs 8.3 months); however, formal between-group comparisons were not conducted. The highest rate of CRs in the study (38% [8/21]) was also observed in patients with cHL receiving combination therapy. The ORR observed with itacitinib plus INCB040093 in this patient subgroup is within the range of response rates (65%-78%) reported for heavily pretreated patients with cHL receiving brentuximab vedotin or the programmed death-1 inhibitors nivolumab and pembrolizumab.34-36 Given this, further study of combined PI3Kδ and JAK1 inhibition in cHL is warranted, and more research is needed to understand the JAK/STAT pathway in this type of lymphoma. Of note, other PI3K inhibitors produce little response in cHL and no known mechanism for the role of JAK1 exists in this lymphoma type (epigenetic changes are thought to be induced by JAK2).37 In addition, the apparent potential of a JAK1 inhibitor to mitigate AST/ALT AEs associated with itacitinib might support combination studies of JAK1 and PD-1 inhibitors, which are known to produce immune-mediated hepatic toxicities, albeit of low severity.38
For patients with DLBCL failing curative therapy, or for those not eligible for stem cell transplant, options remain very limited and without a standard of care. Six patients responded to the combination (for a 26% ORR in DLBCL), including 4 patients with non-GCB DLBCL (for a 31% ORR in non-GCB DLBCL). Among those with DLBCL, the longest DOR (>8.5 months; ongoing at data cutoff) occurred in 2 of the 4 non-GCB DLBCL responders. The small numbers cannot be considered definitive, but the results are in line with in vitro and in vivo data, suggesting a critical role for both PI3K and JAK1 signaling in non-GCB DLBCL, as well as marked potency with dual-target inhibition.13,39
Overall, both monotherapy and combination therapy demonstrated a potential for long-lasting responses. Four patients had responses that lasted longer than 2 years: 1 CR in a patient with FL and 1 PR in a patient with WM receiving monotherapy, and 2 CRs in 2 patients with cHL receiving combination therapy; 3/4 responses were ongoing at data cutoff.
In conclusion, in these heavily pretreated patients with R/R B-cell lymphomas, the PI3Kδ inhibitor INCB040093 alone or in combination with the JAK1 inhibitor itacitinib demonstrated a manageable safety and tolerability profile and encouraging preliminary efficacy. Particularly noteworthy is the activity of the combination treatment in cHL, although no formal comparison was made and no conclusion can be drawn regarding the difference between monotherapy and combination therapy. The results of this study demonstrate the feasibility of targeting both JAK1 and PI3Kδ pathways and serve as proof of concept for future studies of such combination treatments.
Presented in poster/oral presentation form (earlier data cuts) at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland; 17-20 June 2015; 51st Annual Meeting of the American Society of Clinical Oncology, Chicago, IL; 29 May-2 June 2015; and the 20th Congress of the European Hematology Association, Vienna, Austria; 11-14 June 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this study and the investigators and teams who conducted the study. The authors would also like to acknowledge the contributions of Vicky Yuan (Incyte Corporation), who provided statistical programming support; Jennifer Kelley and Jessica Schwartz (Incyte Corporation), who provided operational support; and Peter Langmuir (Incyte Corporation) who provided medical input and guidance in the development of the manuscript. Medical writing assistance was provided by Karolina Rzeniewicz (Evidence Scientific Solutions, London, United Kingdom), and funded by Incyte Corporation.
This study was supported by Incyte Corporation (Wilmington, DE).
Authorship
Contribution: T.J.P., A.F.-T., T.S., C.S.D., P.J., M.T., J.P., L.Z., P.S., and P.M.B. were involved in the conception/design of the work; T.J.P., A.F.-T., T.S., C.S.D., P.J., M.T., P.S., and P.M.B. acquired the data; L.Z., P.S., and X.C. analyzed the data; and all authors drafted the manuscript or revised it critically for important intellectual content.
Conflict-of-interest disclosure: T.J.P. had a consulting/advisory role for Pharmacyclics and Seattle Genetics and received travel/accommodation expenses from Incyte; A.F.-T. participated in a speakers’ bureau for Seattle Genetics and received institutional research funding from Daiichi Sankyo, Genentech/Roche, Gilead Sciences, Immunomedics, Novartis, Oncothyreon, Pfizer, Seattle Genetics, Syndax, and TRACON Pharma. T.S. had a consulting role for Janssen. C.S.D. received research funding from Incyte and had a consulting role for Janssen and Seattle Genetics. P.J. has nothing to disclose. M.T. received research funding from Ariad, Incyte, Novartis, Pfizer, and Sanofi. J.P. is an employee of and has stock ownership with Incyte. L.Z. is an employee of and has stock ownership with Incyte. P.S. is an employee of and has stock ownership with Incyte. X.C. is an employee of and has stock ownership with Incyte. P.M.B. had a consulting role for Gilead and Verastem.
Correspondence: Tycel J. Phillips, Division of Hematology and Oncology, University of Michigan, 1500 E Medical Center Dr, Ann Arbor, MI 48109; e-mail: tycelp@med.umich.edu.

![Figure 5. Best percentage change from baseline in target lesion size in patients with cHL receiving therapy with PI3Kδ inhibitor INCB040093 alone or in combination with JAK1 inhibitor itacitinib. Included are patients with data available for best percentage change from baseline in target lesion size (n = 37 [monotherapy, n = 17; combination therapy, n = 20]). The upper dotted line corresponds to criteria for PD (at least 50% increase) and lower to PR (at least 50% decrease). PD indicates PD or relapsed disease (after CR). Patients with 18F-FDG-avid lymphoma who were PET-positive at baseline could achieve a CR without complete resolution of the target lesion if the lesion became PET-negative per the Revised Response Criteria for Malignant Lymphoma.23 *Best percentage change from baseline in target lesion size >100%. †Patients who discontinued INCB040093 monotherapy and re-enrolled into the study to receive combination therapy. ‡Patients who had previously received INCB040093 monotherapy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/3/10.1182_blood-2017-10-812701/4/m_blood812701f5.jpeg?Expires=1769107604&Signature=klS4hJIslo2cgcyL1n7V0KNF8QTvvpzyVtJ00whrAoRbSL8XznPzlBN5VhcqJhGMYW4aCsOen3fPqi5VCRqnLrugi7yRaBF55mBpZUXtAfUWSYg0HPXAYINKoTJNdxMoU95~2DLcExZOqWde34KmPR0fhJLc1KFLNlmAw9G6b6xqUwEwvQ8gJUq2WYVAUMf-rnCnSMoJkSC6rCxQx1a~kzeVsbBdv3TnYW5KvRxFMkeTW~~BTDmtQhqtIoci0X71WtlyJVJwq6OD1brIEPs0kLlHTGZcXaiD9SYDGojaRnNk3FTYRCKWR83FYY~JmZFwsR5Slh~WmkXhDe3nImt0QA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal