In this issue of Blood, Xu et al unequivocally show by using a series of elegant experiments that platelet GPIbα mediates hepatic thrombopoietin (TPO) production.1
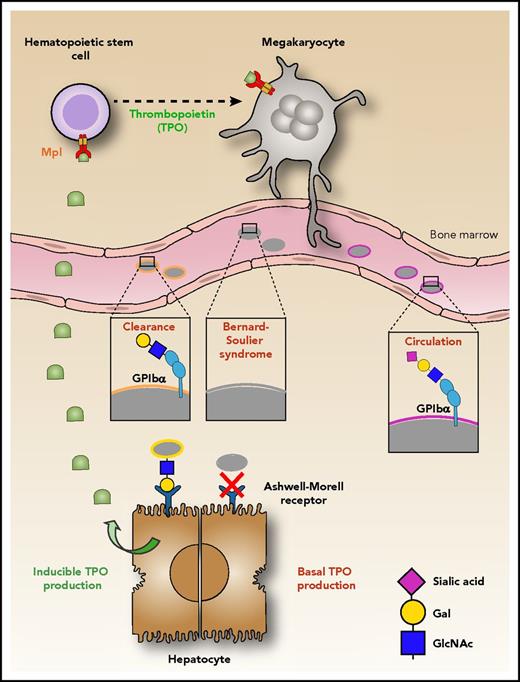
Platelet production begins in the bone marrow where the maturation of megakaryocytes, the platelet precursor cells, is driven by binding of TPO to its receptor, Mpl. Young platelets released in the circulation contain the GPIbα receptor in its fully glycosylated form, in which sialic acid (magenta diamond) covers galactose (yellow circle). Loss of sialic acid (desialylation) on GPIbα induces platelet clearance by the AMR on hepatocytes, thereby inducing the production of hepatic TPO. Xu et al show that platelets lacking GPIbα (as in Bernard-Soulier syndrome) are not recognized by the AMR; hence the production of TPO is reduced.1 Illustration by Leonardo Rivadeneyra, Blood Center of Wisconsin.
Platelet production begins in the bone marrow where the maturation of megakaryocytes, the platelet precursor cells, is driven by binding of TPO to its receptor, Mpl. Young platelets released in the circulation contain the GPIbα receptor in its fully glycosylated form, in which sialic acid (magenta diamond) covers galactose (yellow circle). Loss of sialic acid (desialylation) on GPIbα induces platelet clearance by the AMR on hepatocytes, thereby inducing the production of hepatic TPO. Xu et al show that platelets lacking GPIbα (as in Bernard-Soulier syndrome) are not recognized by the AMR; hence the production of TPO is reduced.1 Illustration by Leonardo Rivadeneyra, Blood Center of Wisconsin.
The work by Xu et al challenges the prevailing model, which posits that TPO production by hepatocytes is constitutive and circulating TPO concentration is inversely proportional to the “Mpl mass” contributed by the total number of megakaryocytes and platelets. Decades of research support the notion that TPO production is constitutive and TPO serum levels are maintained solely by its uptake and metabolism by platelets and megakaryocytes.2 Clear evidence supports this reciprocal relationship between platelet count and circulating TPO levels, for example in bone marrow transplant patients, in Mpl−/− mice, and in models of acute immune or chemotherapy-induced thrombocytopenia.
However, human and mouse data support another TPO regulatory model where platelets are sensed to regulate circulating TPO levels. For example, serum TPO levels are lower than expected in patients with immune thrombocytopenia and high in patients with essential thrombocythemia.3 Moreover, serum TPO levels are normal in Nfe2−/− mice despite severe thrombocytopenia, and mice deficient for Bak and Bax have normal to slightly increased serum TPO levels despite significantly increased platelet count.3 Hence, these data support the notion that platelet TPO metabolism is not the sole determinant of plasma TPO levels regulated in a complex manner.
Recent data give credence to the assertion that TPO regulation is far more complex than previously known. Studies clearly show that the proinflammatory cytokine interleukin-6 (IL-6) stimulates hepatic TPO synthesis, providing a regulated pathway to increase platelet production during acute inflammatory responses in humans and in in vitro models.3 Recent investigations identified that asialylated senile platelets and the hepatocyte-specific Ashwell-Morell receptor (AMR) are the long elusive physiological ligand-receptor pair regulating hepatic TPO messenger RNA (mRNA) production by recruiting JAK2 and STAT3.4 This finding is further supported by data showing that platelets lacking the sialyltransferase ST3GalIV (St3gal4−/−) platelets are exclusively and completely cleared by the AMR.4
GPIbα has been identified as a major counterreceptor on St3gal4−/− platelets for the AMR.5 However, this conclusion was only indirectly supported by enzymatic removal of the N-terminal region of GPIbα from the platelet surface. Xu et al unequivocally show by using a series of elegant experiments that platelet GPIbα mediates TPO production. This conclusion is based on the following data: (1) circulating TPO is significantly decreased in GPIbα-deficient Bernard-Soulier syndrome patients and in Gp1b−/− mice, compared with controls (see figure); (2) lower TPO levels in GPIbα-deficient conditions were not attributable to increased TPO clearance by platelets lacking GPIbα, but rather via decreased hepatic TPO mRNA transcription and production. This conclusion was nicely supported by the fact that wild type, but not Gp1b−/− platelet transfusions rescued both hepatic TPO mRNA and circulating TPO levels in Gp1b−/− mice. Further, in vitro hepatocyte cocultures with platelets or GPIbα-coupled beads confirmed the disruption of platelet-mediated hepatic TPO generation in the absence of GPIbα.
Recent studies have highlighted the role of glycan modifications on platelets in mediating their clearance.3 In circulation, loss of terminal sialic acid (neuraminic acid) from the platelet surface has been linked with senescent platelet removal.4 The evidence provided by Xu et al that GPIbα glycans are recognized by the AMR to initiate hepatic TPO production is somewhat indirect. The authors show that although treatment of Gp1b−/− platelets with neuraminidase caused significant desialylation, desialylated Gp1b−/− platelets failed to rescue impaired hepatic TPO production both in vivo and in vitro. The data support the notion that GPIbα, independent of other receptors and platelet desialylation, is a prerequisite for hepatic TPO production. Additional evidence in support of this notion was provided by recapitulating impaired hepatic TPO production in IL-4Rα/GPIbα transgenic mice, which lack the extracellular GPIbα domain, as well as with antibodies targeting extracellular portions of GPIbα. These results demonstrated that the N-terminus of GPIbα is required for platelet-mediated hepatic TPO generation.
Platelet GPIbα is abundantly decorated with sialic acid moieties, accounting for perhaps 70% to 80% of the total sialic acid on the platelet surface. However, unlike human GPIbα, the murine GPIbα amino acid sequence lacks any N-glycosylation consensus sequences. Grewal et al demonstrated that even in mice lacking GPIbα, neuraminidase treatment leads to efficient platelet clearance, albeit at a slower rate than in control mice.6 The data suggest that the glycans on GPIbα are necessary to set off a rapid rate of AMR-dependent clearance, but the exposed galactose moieties on other platelet glycoproteins present counterreceptors for the AMR or other asialoglycoprotein receptors.
The AMR presents a complex receptor that prefers complex N-linked glycans that are clearly absent from mouse platelets on GPIbα.7 Hence, other binding partners could be involved in AMR-mediated platelet clearance. For example, platelet bound von Willebrand factor, the ultimate GPIbα ligand, which expresses multiple N- and O-linked glycans in human and mice, could clearly contribute to the AMR-platelet recognitions.8 It is unclear whether asialylated GPIbα O-linked glycans contribute to AMR-mediated platelet clearance. A recent study demonstrated that hepatic AMR promotes preferential adherence to and phagocytosis of asialylated and/or platelets that lack O-glycans, that is, platelets from hematopoietic cell conditional C1galt1−/− mice, by Kupffer cells through the C-type lectin receptor CLEC4F.9 These findings provide further insight into an essential role for core 1 O-glycosylation of platelets in their clearance in the liver. It remains to be determined if removal of sialic acid moieties (which come in different flavors) from O-linked glycans play a role in AMR-platelet recognition. These intriguing observations require further investigation to fully understand the contribution of the published phenomena to platelet clearance and TPO production.
Despite the limitations of the here published findings, the data reveal a novel nonredundant regulatory role of platelets in hepatic TPO homeostasis, which furthers our understanding of TPO regulation and may have important implications in diseases related to GPIbα such as Bernard-Soulier syndrome and auto- and alloimmune-mediated thrombocytopenias.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal