In this issue of Blood, Dai et al demonstrate a dynamic interchange of cell surface–bound platelet factor 4 (PF4) among hematopoietic and vascular cells that may limit the thrombocytopenia and promote prothrombotic processes in heparin-induced thrombocytopenia (HIT).1
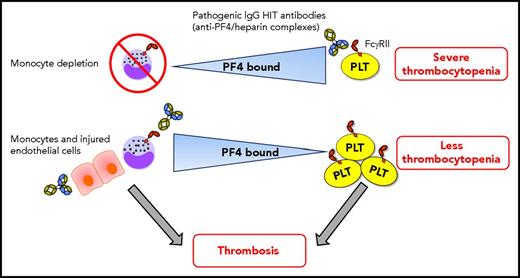
Dynamic intercellular PF4 redistribution may modulate thrombocytopenia and thrombosis immune responses in HIT. When monocytes are depleted, platelets bind more PF4, which results in severe HIT immunoglobulin G (IgG) antibody (with specificity for PF4/heparin complexes) -mediated thrombocytopenia. The amount of PF4 bound per platelet varies inversely with the WBC:platelet ratio, predominantly due to monocytes, which have a strong binding affinity for PF4. HIT antibodies potently promote prothrombotic pathways via triggering of monocyte FcγRIIA. PF4 also translocates effectively from platelets toward to the injured/inflamed endothelium that may also promote prothrombotic processes in HIT. Shuttling of PF4 from platelets toward monocytes (and perhaps endothelial cells) mitigates the degree of thrombocytopenia. Consequently, more platelets may be available in circulation for contributing to the development of thrombosis. Note: HIT antibodies also target platelet FcγRIIA, activating platelets and stimulating the release of procoagulant microparticles, which can also contribute to thrombosis. This is not depicted in the figure for simplicity. PLT, platelet.
Dynamic intercellular PF4 redistribution may modulate thrombocytopenia and thrombosis immune responses in HIT. When monocytes are depleted, platelets bind more PF4, which results in severe HIT immunoglobulin G (IgG) antibody (with specificity for PF4/heparin complexes) -mediated thrombocytopenia. The amount of PF4 bound per platelet varies inversely with the WBC:platelet ratio, predominantly due to monocytes, which have a strong binding affinity for PF4. HIT antibodies potently promote prothrombotic pathways via triggering of monocyte FcγRIIA. PF4 also translocates effectively from platelets toward to the injured/inflamed endothelium that may also promote prothrombotic processes in HIT. Shuttling of PF4 from platelets toward monocytes (and perhaps endothelial cells) mitigates the degree of thrombocytopenia. Consequently, more platelets may be available in circulation for contributing to the development of thrombosis. Note: HIT antibodies also target platelet FcγRIIA, activating platelets and stimulating the release of procoagulant microparticles, which can also contribute to thrombosis. This is not depicted in the figure for simplicity. PLT, platelet.
HIT is an immune complication of therapy with heparin characterized by thrombocytopenia and increased risk of thrombosis, the most dreaded complication of HIT responsible for the majority of the morbidity and mortality.2 HIT is triggered by antibodies that recognize complexes of PF4 and heparin.3 The cellular target for the HIT antibodies is platelets, resulting in antibody-mediated platelet destruction leading to thrombocytopenia. Furthermore, HIT antibodies may also target platelet FcγRIIA,4 causing platelet activation and the release of procoagulant microparticles that may contribute to thrombosis.5 Moreover, cellular activation via monocyte FcγRIIA has been shown to be a trigger for intense thrombin generation and thrombosis in HIT.6 HIT immune complexes not only form on the surface of monocytes but also form on endothelial cells expressing glycosaminoglycans, further contributing to prothrombotic processes.7 Using a murine passive immunization model of HIT, it was previously shown that monocyte depletion attenuated thrombosis but surprisingly worsened the degree of thrombocytopenia in the mice.8 In the current study, the authors test this mechanism in HIT, focusing on cellular PF4 binding. They report that the redistribution of PF4 from platelets to monocytes (and perhaps endothelium) lessens the severity of the thrombocytopenia in HIT, leaving an increased number of platelets in the circulation that may possibly contribute to development of thrombosis. Monocytes bind PF4 with a greater affinity than other cell types, including platelets as was shown in vitro as well as in vivo using the murine passive immunization model of HIT. In vivo, HIT induction caused a transient monocytopenia, which normalized followed by an increase in the platelet count. Interestingly, it was also observed that the red blood cell (RBC) pool contained the most bound PF4; however, the surface-bound PF4 level per cell was low. This suggests a potential role for the RBC pool as a low-affinity reservoir for PF4 in the circulation. To further assess the dynamic nature of PF4, white blood cell (WBC) to platelet ratios were altered, and it was shown that the amount of PF4 bound per platelet varied inversely with the WBC:platelet ratio. When monocytes were depleted, platelets bound more PF4. Subsequently, the redistribution of PF4 between hematopoietic and vascular endothelial cells was investigated. Using microfluidic channels coated with endothelial cells, it was demonstrated that PF4 translocated more effectively from platelets than from WBCs to the endothelium. The severity of trauma, which includes both vascular and inflammatory components, has been shown to correlate with the immune response in HIT patients.9 The authors therefore investigated the effect of endothelial injury using microfluidic channels and observed increased binding of HIT antibodies with injured endothelial cells compared with resting endothelial cells. Thus, PF4 distribution on the endothelial cell surface is dependent on the vascular lining and endothelial activation.
Dai and colleagues have convincingly demonstrated that shuttling of PF4 occurs between diverse hematopoietic and vascular endothelial cell surfaces and that these dynamics are strongly dependent on monocytes, which have a high binding affinity for PF4, on alterations in cell counts, and on endothelial cell injury/inflammation. Their findings suggest that redistribution of PF4 away from platelets may mitigate the severity of thrombocytopenia, which may paradoxically allow those platelets to be involved in thrombosis (see figure). However, how PF4 redistribution among different cell types functionally regulates the development of thrombocytopenia and thrombosis formation has yet to be determined. Subsequent studies should explore these functional effects of PF4. How is intercellular PF4 redistribution affecting the coagulation cascade, and what is the effect on the activation status of monocytes during the immune response in HIT? Is there a role for monocytes as antigen-presenting cells in HIT? The potential role for RBCs as a low-affinity reservoir for PF4 in circulation should also be further investigated.
In summary, the current paper significantly enhances our understanding of the HIT pathogenesis, and follow-up studies will provide further insights into the role of PF4 redistribution in orchestrating thrombocytopenia and thrombotic pathways in HIT.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal