Abstract
High-risk chronic lymphocytic leukemia (CLL) has been defined by clinical and/or genetic resistance (TP53 abnormalities) to treatment with chemoimmunotherapy (CIT). With the availability of pathway inhibitors (PIs), such as kinase inhibitors and BCL2 antagonists, the outlook of CIT-resistant patients has dramatically improved. Here, we propose a revision of the concept of high-risk CLL, driven by TP53 abnormalities and response to treatment with PI. CLL high-risk-I, CIT-resistant is defined by clinically CIT-resistant disease with TP53 aberrations, but fully responsive to PI. This category is largely the domain of PI-based therapy, and cellular therapy (ie, allogeneic hematopoietic cell transplantation) remains an option only in selected patients with low individual procedure-related risk. In CLL high-risk-II, CIT- and PI-resistant, characterized by increasing exhaustion of pharmacological treatment possibilities, cellular therapies (including chimeric antigen receptor-engineered T cells) should be considered in patients eligible for these procedures. Moreover, molecular and cellular therapies are not mutually exclusive and could be used synergistically to exploit their full potential.
Introduction
Chronic lymphocytic leukemia (CLL) has been considered as high-risk if 1 or more of the following conditions are met: (1) disease refractory to purine analogs; (2) disease relapsing within 2 years after chemoimmunotherapy (CIT); and (3) disease with deletion and/or mutation of the TP53 gene.1-4 Recently, pathway inhibitors (PIs), such as inhibitors of Bruton tyrosine kinase (BTKis), phosphatidylinositol 3 kinase (PI3Kis), and BCL2 (BCL2is), have dramatically improved treatment options for patients with high-risk CLL.
In 2014, our 2 societies published some guidance for counseling patients with high-risk CLL at a time point when experience with PIs was limited.5 Since then, PIs became broadly available, and long-term data on treatment results are emerging. Moreover, chimeric antigen-receptor-engineered T cells (CAR T cells) have entered the stage as a novel form of targeted cellular immunotherapy (CI) for B-cell malignancies, including CLL.
Given these potent novel treatment options, the traditional CIT-based high-risk definition might be no longer appropriate for identifying CLL patients who are in need for more aggressive or experimental therapy. Here we propose a reformulation of the criteria defining high-risk CLL along with a treatment algorithm according to this new definition. For reasons of practicability, only approved PIs have been taken into account.
Current evidence
Pathway inhibitors
BTKi
Ibrutinib is the only BTKi currently approved for CLL.
Efficacy
The reported overall response rates (ORRs) to ibrutinib monotherapy in patients with relapsed/refractory (R/R) CLL are excellent (80%-95%), but only ≤10% achieve complete response (CR), and the proportion of patients with clearance of minimal residual disease (MRD) is negligible. Two-year progression-free survival (PFS) and overall survival (OS) estimates are reproducibly between 65% to 80%, and ∼80%, respectively (supplemental Table 1, available on the Blood Web site).6-10 In the study with the longest observation time, 5-year PFS and OS rates were 44% and 57% in 101 patients with R/R CLL, respectively.6,10 However, there is no plateau in the remission duration curves, and ibrutinib continuously needs to be withdrawn because of toxicity, CLL progression, or Richter transformation (RT).9,11 Disease control is much better if ibrutinib is used as first-line therapy in treatment-naïve (TN) patients, with PFS rates >85% at 2 years and beyond,10,12 even in the presence of TP53 alterations, although data on larger number of patients with extended follow-up are needed.13
Prognostic factors
Deletions and/or mutations of the TP53 gene are currently the only accepted predictive biomarkers in CLL. Most patients with TP53 lesions respond poorly to CIT. In contrast, response rates to ibrutinib are not affected by TP53 lesions.14 However, TP53 defects seem to facilitate not only the development of mutations of BTK or PLCg2 conferring ibrutinib resistance,8,15-18 but also of alternative mutations driving clonal evolution.19 Patients with TP53 lesions have shorter remission duration if treated with ibrutinib in the R/R setting (2-year PFS,55%-75%) (Table 1).6,7,10,13,20,21
Results of approved pathway inhibitors in R/R CLL with TP53 abnormalities and/or complex karyotype
| Agent (study) . | Study type . | Aberration . | n . | Age in years (range) . | ORR (CR)* . | DOR (2 y) (median in mo) . | PFS (2 y) (median in mo) . | OS (2 y) (median in mo) . | Median follow-up, mo (range) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Ibrutinib (PCYC-1102/1103) | Prospective | 17p− | 34 | 79% (6%) | 31 mo | 57% (26) | 64% (57) | 47 (1-67) | 10 | |
| CK | 37 | 90% (7%) | 39 mo | 65% (33) | 75% (57) | 55 (1-67) | ||||
| Ibrutinib + rituximab | Prospective | 17p− | 21 | 86% (24%) | NA | 55% (32) | 73% (NR) | 47 (36-51)† | 7 | |
| CK | 15 | NA | NA | 55% (32) | 77% (NR) | |||||
| Ibrutinib (NCT01500733) | Prospective | 17p− | 16 | 62 (33-82)† | NA | NA | 74% (39) | 81% (63) | 57† | 13 |
| Ibrutinib (PCYC-1117 / RESONATE17) | Prospective | 17p− | 144 | 64 (IQR 57-72) | 83% (8%) | 70% (NR) | 63% (NR) | 75% (NR) | 28 (IQR 15-28) | 33 |
| Ibrutinib ± rituximab ± bendamustine | Retrospective | 17p− | 34 | NA | NA | 55% (32) | 65% (33) | 28† (14-48) | 20 | |
| CK | 21 | NA | NA | 25% (19) | 55% (25) | |||||
| Ibrutinib CLL Connect USA | “Real-world” | 17p− | 123 | 62 (35-80)† | NA | NA | 64% (36) | NA | 17 (1-60)† | 28 |
| CK | 96 | NA | NA | 55% (29) | NA | |||||
| Idelalisib + rituximab-bendamustine | Prospective | 17p−/ TP53mut | 69 | 62 (38-83)† | 58% (0%) | NA | 29% (11) | NA (NR) | 14 (IQR 7-18)† | 39 |
| Idelalisib + rituximab (0116 trial karyotyped) | Prospective | CK | 26 | 69 (58-84) | 81% (0%) | NA (21) | NA (NR) | 21 | 40 | |
| Idelalisib CLL Connect USA | “Real-world” | 17p− | 17 | 62 (35-80)† | NA | NA | 20% (12) | NA | 17 (1-60)† | 28 |
| CK | 12 | NA | NA | 20% (9) | NA | |||||
| Venetoclax M13-982 extension | Prospective | 17p−/TP53mut | 153 | 67 (29-85) | 77% (18%) | 65% (33) | 53% (26) | 72% (39) | 23 (0-44)‡ | 48 |
| Venetoclax 400mg M12-175, M14-032, M13-982, M13-365 pooled | Pooled prospective | 17p−/ TP53mut | 152 | 76% (17%) | 63% (27) | 51% (25)§ | NA | 16 (0-54)‡ | 16 |
| Agent (study) . | Study type . | Aberration . | n . | Age in years (range) . | ORR (CR)* . | DOR (2 y) (median in mo) . | PFS (2 y) (median in mo) . | OS (2 y) (median in mo) . | Median follow-up, mo (range) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Ibrutinib (PCYC-1102/1103) | Prospective | 17p− | 34 | 79% (6%) | 31 mo | 57% (26) | 64% (57) | 47 (1-67) | 10 | |
| CK | 37 | 90% (7%) | 39 mo | 65% (33) | 75% (57) | 55 (1-67) | ||||
| Ibrutinib + rituximab | Prospective | 17p− | 21 | 86% (24%) | NA | 55% (32) | 73% (NR) | 47 (36-51)† | 7 | |
| CK | 15 | NA | NA | 55% (32) | 77% (NR) | |||||
| Ibrutinib (NCT01500733) | Prospective | 17p− | 16 | 62 (33-82)† | NA | NA | 74% (39) | 81% (63) | 57† | 13 |
| Ibrutinib (PCYC-1117 / RESONATE17) | Prospective | 17p− | 144 | 64 (IQR 57-72) | 83% (8%) | 70% (NR) | 63% (NR) | 75% (NR) | 28 (IQR 15-28) | 33 |
| Ibrutinib ± rituximab ± bendamustine | Retrospective | 17p− | 34 | NA | NA | 55% (32) | 65% (33) | 28† (14-48) | 20 | |
| CK | 21 | NA | NA | 25% (19) | 55% (25) | |||||
| Ibrutinib CLL Connect USA | “Real-world” | 17p− | 123 | 62 (35-80)† | NA | NA | 64% (36) | NA | 17 (1-60)† | 28 |
| CK | 96 | NA | NA | 55% (29) | NA | |||||
| Idelalisib + rituximab-bendamustine | Prospective | 17p−/ TP53mut | 69 | 62 (38-83)† | 58% (0%) | NA | 29% (11) | NA (NR) | 14 (IQR 7-18)† | 39 |
| Idelalisib + rituximab (0116 trial karyotyped) | Prospective | CK | 26 | 69 (58-84) | 81% (0%) | NA (21) | NA (NR) | 21 | 40 | |
| Idelalisib CLL Connect USA | “Real-world” | 17p− | 17 | 62 (35-80)† | NA | NA | 20% (12) | NA | 17 (1-60)† | 28 |
| CK | 12 | NA | NA | 20% (9) | NA | |||||
| Venetoclax M13-982 extension | Prospective | 17p−/TP53mut | 153 | 67 (29-85) | 77% (18%) | 65% (33) | 53% (26) | 72% (39) | 23 (0-44)‡ | 48 |
| Venetoclax 400mg M12-175, M14-032, M13-982, M13-365 pooled | Pooled prospective | 17p−/ TP53mut | 152 | 76% (17%) | 63% (27) | 51% (25)§ | NA | 16 (0-54)‡ | 16 |
Only studies for which 24-mo estimates were available were considered.
NA, not available; NR, not reached; prosp, prospective; retrosp, retrospective.
Overall response including partial response with persistent lymphocytosis.
Data for the whole sample including patients without TP53 aberrations/complex karyotype.
Time on venetoclax.
Including patients with other doses (150-1200 mg).
Deletion 11q was associated with inferior PFS in the pivotal ibrutinib trial,6 but this could not be confirmed in subsequent studies.7,8,22 More recently, complex karyotype (CK) has been rediscovered as an adverse factor for CLL outcome.22-24 Although there are data suggesting that CK in the absence of TP53 aberrations does not affect duration of response to ibrutinib,10,22 CK may augment the adverse effect of TP53 lesions on response duration.17,20 Data on the impact of clinical parameters, such as age and pretreatment lines, on duration of response to ibrutinib are still inconsistent.10,17,20,25 Dose adherence appears to be crucial for ibrutinib treatment success.26
Safety
The most important adverse events (AEs) resulting in treatment discontinuation are infections/pneumonitis, atrial fibrillation, and bleeding events, each accounting for up to 25% of all AE-related discontinuations.27-29 Atrial fibrillation risk may be less critical with a second-generation BTKi, such as acalabrutinib.30,31 In addition, ventricular arrhythmias on ibrutinib have been reported.32 In clinical trials, the proportion of patients dying on therapy (not necessarily related to therapy) in the absence of CLL progression has been consistently small (5%-10%).6-8,33 The risk of treatment-emergent autoimmune hemolytic anemia is low.34 Recent reports suggest an increased risk of early-onset invasive fungal infections on ibrutinib.35,36
PI3Ki
Idelalisib, a PI3Kdelta inhibitor, is approved in combination with rituximab or ofatumumab for treatment of CLL.
Efficacy
In patients with R/R CLL, idelalisib shows response rates similar to ibrutinib, but with shorter median PFS (generally <2 years), even if combined with bendamustine (supplemental Table 1).37-39
Prognostic factors
Safety
The most relevant grade ≥3 toxicities of idelalisib in the R/R setting consist in infections and autoimmune-mediated inflammations such as enteritis/diarrhea (≤20%), transaminitis (≤15%), and pneumonitis (≤5%).41 Moreover, opportunistic infections have been observed,42 making longitudinal monitoring of cytomegalovirus reactivation and Pneumocystis jiroveci pneumonia prophylaxis mandatory. Preliminary analyses suggest a 2-year risk of fatal AE for idelalisib-based therapies of ≥10% in patients with R/R CLL.39,42
BCL2i
Venetoclax is a BCL2i approved for patients with CLL with TP53 deletion unsuitable for BTKi/PI3Ki, and those who have failed both CIT and BTKi or PI3Ki.
Efficacy
Although ORR (70%-80%) to venetoclax are not superior to those of BTKi/PI3Ki, a sizable proportion of patients (20%-30%) achieve CR and even MRD negativity in the R/R setting, which might be further increased if venetoclax is combined with rituximab (supplemental Table 1).16,43-46 Although these patients may enjoy prolonged disease control, median response duration is <30 months in patients not reaching CR or MRD negatitvity.16,45,47,48
Prognostic factors
Similar to ibrutinib, venetoclax shows high response rates but decreased remission duration if TP53 abnormalities are present (Table 1).16,43,44,49 Recent data suggest that CK, prior PI exposure, multiple pretreatment lines, and bulky disease are additional risk factors for venetoclax failure.49-52 In a retrospective analysis, prior PI, TP53 abnormalities, CK, and prior CI were associated with inferior PFS on venetoclax.49 Preliminary data on mutations driving venetoclax resistance suggest a complex pattern of clonal evolution,53 precluding their use as biomarker for venetoclax treatment success.
Safety
Although cases of fatal tumor lysis syndrome were initially reported, this problem has been virtually eliminated by the introduction of ramp-up dosing strategies and strict tumor lysis prophylaxis. Grade 3/4 neutropenia and thrombopenia develop in up to 40% and 15% of the patients, respectively.43,44 In addition, serious infections can occur during venetoclax treatment, but the rate of fatal treatment-emergent AE was <5% in the published trials.43,44,48
Pathway inhibitor resistance: secondary treatment options and outcome
Basically there are 3 types of PI failure: (1) discontinuation because of toxicity; (2) CLL progression; and (3) RT. The relatively high proportion of early RT events in R/R patients on PI has raised concerns that PI themselves might induce transformation. Although an impact of PI on the microenvironmental competition between CLL and Richter clones cannot be excluded,18 this phenomenon might be explained by the fact that PI act as a “filter” for preexisting subclinical RT by suppressing aggressive untransformed CLL otherwise limiting the patient’s prognosis. It remains to be shown if intrinsic effects of PI, such as triggering of genomic instability in B cells,54 may contribute to RT development. In contrast to BTKi/PI3Ki discontinuation because of AE (which account for one-half of all discontinuations in R/R patients within the first 2 years38,55 ), the outcome of BTKi/PI3Ki failure from disease progression or transformation has been generally poor, with median OS times below 30 months for CLL and a few months for RT.17,55-58 Information on the outcome of patients failing venetoclax is sparse. In a prospective trial enrolling 17p-deleted patients, 14 of 22 patients with disease progression died within a year after venetoclax discontinuation.44 Similarly, the median OS of 25 patients progressing on venetoclax trials performed in Australia was 13 months.50
Because the results of CIT-based salvage regimens are poor after CLL progression on BTKI/PI3Ki given for R/R disease,57 treatment revolves around alternative PI treatment. For ibrutinib failure, the reported response rates with idelalisib and venetoclax were 28% to 46% and 61% to 76%, respectively.38,47,48,57 Likewise, PFS seems to be substantially shorter with idelalisib than with venetoclax in that setting.38 In a prospective study on venetoclax as rescue strategy for ibrutinib resistance, an objective response was achieved in 65% (CR, 9%) of patients and the median PFS was 25 months.47 A recent “real-world” analysis reported significantly reduced PFS on venetoclax in patients with prior PI failure.49
Conversely, ORR to ibrutinib after idelalisib failure are more encouraging with 64% to 76% observed in preliminary studies,38,57 similar to those obtained with venetoclax (Table 2).59 However, information about alternative PI treatment comes from small studies with limited follow-up. Novel PIs such as indirect BTKi, C481S-independent BTKi, and PKCβ inhibitors may gain a role in management of primary PI resistance,11 but none of these agents have reached the clinical stage yet.
Results of secondary pathway inhibitors in relapsed/refractory CLL
| First PI (study) . | N (% 17p−) (% CK) . | Age, y (range) . | Cause of failure of first PI . | Second PI (n) . | ORR to second PI, % . | Outcome of second PI . | Median follow-up, mo (range) . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Ibrutinib Idelalisib (US Real World) | 143 (37% 17p−) (33% CK) 35 (24% 17p−) (18% CK) | 60 (33-89) | Toxicity, 51% CLL, 29% Other, 20% | Idelalisib (22) Ibrutinib (16) Venetoclax (13) Untreated despite CLL progression/RT (10/59) | 28 64 76 | PFS 9 mo (If CLL was cause of first PI failure) PFS NR (if toxicity was cause of first PI failure) | 14 (0.3-51) (From first PI initiation) | 57 |
| Ibrutinib (M14-032) | 91 (47% 17p−) | 66 (28-81) | CLL, 100% | Venetoclax (91) | 65 (CR, 9) | PFS 25 mo | 14 | 47 |
| (IQR 8-18) | ||||||||
| (From second PI initiation) | ||||||||
| Idelalisib (M14-032) | 36 (22% 17p−) | 68 (56-85) | CLL, 100% | Venetoclax (36) | 67 (CR, 9) | 1-y PFS, 79% | 59 | |
| BCR inhibitor (M13-982) | 16 (100% 17p−) | NA | PD, 14; AE, 2 | Venetoclax (16) | 63 (CR, 13) | 2-y PFS, 50% | 16 (1-49)* | 48 |
| 2-y OS, 55% | ||||||||
| Venetoclax | 25 (40% 17p−) | 62 (47-78) | CLL, 32% RT, 68% | Ibrutinib (6) Ibrutinib (4, for CLL progression subsequent to RT treatment | 83 100 | OS after venetoclax failure CLL, 9 mo RT, 12 mo | NA | 50 |
| First PI (study) . | N (% 17p−) (% CK) . | Age, y (range) . | Cause of failure of first PI . | Second PI (n) . | ORR to second PI, % . | Outcome of second PI . | Median follow-up, mo (range) . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Ibrutinib Idelalisib (US Real World) | 143 (37% 17p−) (33% CK) 35 (24% 17p−) (18% CK) | 60 (33-89) | Toxicity, 51% CLL, 29% Other, 20% | Idelalisib (22) Ibrutinib (16) Venetoclax (13) Untreated despite CLL progression/RT (10/59) | 28 64 76 | PFS 9 mo (If CLL was cause of first PI failure) PFS NR (if toxicity was cause of first PI failure) | 14 (0.3-51) (From first PI initiation) | 57 |
| Ibrutinib (M14-032) | 91 (47% 17p−) | 66 (28-81) | CLL, 100% | Venetoclax (91) | 65 (CR, 9) | PFS 25 mo | 14 | 47 |
| (IQR 8-18) | ||||||||
| (From second PI initiation) | ||||||||
| Idelalisib (M14-032) | 36 (22% 17p−) | 68 (56-85) | CLL, 100% | Venetoclax (36) | 67 (CR, 9) | 1-y PFS, 79% | 59 | |
| BCR inhibitor (M13-982) | 16 (100% 17p−) | NA | PD, 14; AE, 2 | Venetoclax (16) | 63 (CR, 13) | 2-y PFS, 50% | 16 (1-49)* | 48 |
| 2-y OS, 55% | ||||||||
| Venetoclax | 25 (40% 17p−) | 62 (47-78) | CLL, 32% RT, 68% | Ibrutinib (6) Ibrutinib (4, for CLL progression subsequent to RT treatment | 83 100 | OS after venetoclax failure CLL, 9 mo RT, 12 mo | NA | 50 |
Bold indicates PFS times.
IQR, interquartile range; NA, not available; NR, not reported; PD, progressive disease.
Time on venetoclax.
PI: open issues
Open issues of PI treatment in high-risk CLL include the efficacy and safety of combining PI with each other or CIT, the optimum treatment dose and duration, the impact of response depth on outcome, and the economic burden associated with long-term administration of PI. The currently explored approaches of early use of PI combinations appear to be particularly promising and may require further development of the concept of high-risk CLL if they should be established as therapeutic standard. In addition, the long-term toxicities of PI need careful evaluation, including unforeseen caveats such as the potential triggering of genomic instability in B cells by some of these agents.54
Cellular immunotherapy
AlloHCT
Efficacy
The basic principle of allogeneic hematopoietic cell transplantation (alloHCT) is establishing a foreign immune system in the patient for permanent suppression or eradication of recipient lymphohematopoiesis including leukemia stem cells. This effect is called graft-versus-leukemia (GVL) activity. Patients with effective GVL, as indicated by clearing MRD upon immunosuppression withdrawal, have an extremely low risk of disease recurrence. In the prospective CLL3X trial of the German CLL Study Group, the relapse risk of patients following this pattern of GVL-mediated MRD clearance was only 12% at 10 years after alloHCT.60 Similarly, the 5-year MRD recurrence rate was only 6% after GVL-mediated MRD eradication in a single-center study.61 Overall, studies on reduced-intensity conditioning (RIC) alloHCT in CLL show PFS and OS rates of 50% to 60% and 60% to 75% at 2 years and of 35% to 45% and 45% to 65% at 5 years, respectively.61-67 Long-term follow-up studies report 10-year PFS of ∼30% after alloHCT.60,67 In a large European Society for Blood and Marrow Transplantation (EBMT) registry study as well as in the prospective CLL3X trial, PFS at 10 years after alloHCT was 79% for those patients who passed the 5- or 6-year landmark event-free. In conclusion, about 30% of all transplanted patients will durably benefit from a “targeted” GVL effect.
Prognostic factors
TP53 abnormalities have not been associated with inferior outcome after RIC alloHCT in most studies.61-64,68 The impact of CK on alloHCT outcome requires further analysis.64,69,70 The most important risk factor for an adverse transplant outlook is refractory disease at alloHCT,62-64,71,72 with patients transplanted in remission having a better outcome (2-year PFS, 55%-65%).60,61,67,68 In addition to disease-related risk factors, patient- and procedure-related variables, such as age, sex mismatch, donor type, performance status (PS), T-cell depletion, but also center experience73 determine alloHCT outcome. For example, patients <45 years, with good PS and favorable donor-recipient sex constellation who were in remission at alloHCT, had 5-year PFS of 55% to 64% in a large EBMT analysis.68 Similarly, in the CLL3X trial, patients with chemosensitive disease not receiving alemtuzumab as a T-cell depletion method had a PFS of 62% and 46% at 5 and 10 years, respectively.60
Safety and treatment complications
With modern transplantation strategies, the early-death rate of CLL allotransplants (ie, death within the first 100 days after alloHCT) is <5%.5 The good tolerability of RIC alloHCT allows offering the procedure to older subjects and patients with comorbidity. However, largely because of graft-versus-host disease (GVHD)-related complications, nonrelapse mortality (NRM) may increase to >40% at 2 years posttransplant in patients with adverse patient-, donor-, and procedure-related risk factors, as mentioned previously.68,74 In contrast, for patients with a combination of favorable risk factors at alloHCT, the 2-year risk of NRM was 12% in a large registry cohort.68 Apart from its impact on NRM, chronic GVHD is the major determinant affecting quality of life after alloHCT. About 25% of survivors will experience impaired quality of life during the first posttransplant years because of chronic GVHD.62,71,75 Moreover, allografted patients are at a higher risk of mortality, infections, and hospitalization than sex- and age-matched controls over their lifespan.76
Relapse after alloHCT: secondary treatment options and outcome
Already in the CIT era, the prognosis of CLL relapse posttransplant seemed not to be inferior to that of high-risk CLL in untransplanted patients (supplemental Table 2).61,77 The advent of PI, especially ibrutinib, has substantially improved treatment options for high-risk CLL progressing after alloHCT. Recent data suggest that safety and efficacy of ibrutinib given for CLL relapse after allotransplant are as good as in untransplanted patients (Table 3).78-80 Moreover, ibrutinib may enhance Th1-mediated GVL effects if given on a donor chimerism scenario.79 Accordingly, the outcome of posttransplant relapse in CLL patients has substantially improved.
Results of ibrutinib for CLL relapse after alloHCT
| Series (study) . | N (% 17p−) . | Age, y . | Time from alloHCT to ibrutinib, mo (range) . | ORR (CR), % . | Grade 3-5 toxicity . | De novo GVHD . | 2-y PFS, % . | 2-y OS, % . | Median follow-up, mo (range) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dresden | 5 (20% 17p−) | 58 (38-63) | 30 (6-38) | 100 (0) | 40% (infection; no fatalities) | 0 | NA | NA | NA | 78 |
| US trial cohort | 16 (63% 17p−) | 55 (43-68) | 27 (8-115) | 88 (13%) | 75% (infection, bleeding; 2 fatalities) | 0 | 77 | NA | NA | 79 |
| Stanford | 11 (36% 17p−) | 59 (41-69) | 55 (43-68) | 91 (64) | 25% (infection, skin; 2 fatalities) | 0 | NA | NA | NA | 79 |
| EBMT | CLL 55 (31% 17p−) | 55 (38-66) | 21 (0.5-81) | 70 (33) | 10% (second neoplasm, skin; no fatalities) | 1 chronic (limited) | 51 | 72 | 14 (3-32) | 80 |
| MCL 5 |
| Series (study) . | N (% 17p−) . | Age, y . | Time from alloHCT to ibrutinib, mo (range) . | ORR (CR), % . | Grade 3-5 toxicity . | De novo GVHD . | 2-y PFS, % . | 2-y OS, % . | Median follow-up, mo (range) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dresden | 5 (20% 17p−) | 58 (38-63) | 30 (6-38) | 100 (0) | 40% (infection; no fatalities) | 0 | NA | NA | NA | 78 |
| US trial cohort | 16 (63% 17p−) | 55 (43-68) | 27 (8-115) | 88 (13%) | 75% (infection, bleeding; 2 fatalities) | 0 | 77 | NA | NA | 79 |
| Stanford | 11 (36% 17p−) | 59 (41-69) | 55 (43-68) | 91 (64) | 25% (infection, skin; 2 fatalities) | 0 | NA | NA | NA | 79 |
| EBMT | CLL 55 (31% 17p−) | 55 (38-66) | 21 (0.5-81) | 70 (33) | 10% (second neoplasm, skin; no fatalities) | 1 chronic (limited) | 51 | 72 | 14 (3-32) | 80 |
| MCL 5 |
MCL, mantle cell lymphoma; NA, not available.
alloHCT: open issues
Open issues of alloHCT in the PI era are largely related to the interactions of PI and transplantation. Evidence is emerging that PI can safely bridge CIT-refractory patients to transplant.81,82 Whereas data supporting the safety and efficacy of ibrutinib in posttransplant CLL relapse in BTKi-naïve patients is accumulating, the feasibility of posttransplant PI salvage in PI-preexposed patients remains to be shown.80 Furthermore, the use of PI for prevention of early disease recurrence posttransplant warrants investigation. The same accounts for the possible downregulation of GVHD activity by ibrutinib.83 In contrast, information on the posttransplant use of PI3Ki and BCL2i is limited. Moreover, there are only scanty data on the efficacy of alloHCT in patients who have failed a PI.
CAR T cells
Whereas the GVL effect of alloHCT relies on a polyclonal immune reaction against multiple undefined target antigens, CAR T cells exert a monoclonal immune activity against defined antigens, thereby avoiding the GVH reactions linked to alloHCT efficacy.
Published data on CAR T cells in CLL are limited.84 Investigators from the University of Pennsylvania treated 14 patients with heavily pretreated CLL (43% with TP53 abnormality) with the anti-CD19 construct CTL019. Fifty-seven percent of the patients responded, 29% with a CR. All complete responders became durably MRD-negative and developed permanent B-cell aplasia. With 1 patient in CR dying of infection, 18-month PFS was 29%. Grade 3-4 cytokine-release syndrome (CRS) developed in 50% of the patients and correlated with in vivo CTL019 expansion.85 The Fred Hutchison Cancer Research Center published results on 24 patients with R/R CLL having failed ibrutinib who received the anti-CD19 construct JCAR014. A response was observed in 74% of the patients. Of 12 patients tested, 7 were MRD-negative by deep sequencing and remained relapse-free after a median follow-up of 7 months. Grade ≥3 CRS and neurotoxicity occurred in 2 and 6 patients, respectively, with 1 fatality.86
Altogether, CAR T cells seem to have considerable therapeutic potential in CLL, including patients refractory to CIT and PI or relapsed following alloHCT.87 Although further studies and a longer follow-up are needed, durable MRD-negative CR may be achieved in a sizable minority of patients. Ibrutinib given at the time of autologous T-cell collection and/or at the time of CAR T-cell reinfusion may further enhance efficacy.88 Relevant early toxicities consist in CRS and neurologic complications.
Further fine-tuning of constructs, T-cell sources, ex vivo CAR T-cell expansion, dosing, lymphodepletion, and other variables, as well as addressing additional target antigens, will likely improve treatment results of CAR T cells in CLL.84,89 Currently, however, in the absence of a construct approved for CLL, CAR T cells have to be considered as an experimental treatment in this disease. Apart from the various technical aspects mentioned, unsolved issues include long-term efficacy and safety and the economical aspects of this approach.
A new categorization of high-risk CLL
In the CIT era, high-risk CLL has been defined by (1) TP53 lesions, (2) flurabine refractoriness, and (3) early relapse after CIT. With the advent of PI, the outcome of patients meeting any of these 3 criteria has substantially improved. This is particularly true for TN patients with TP53 abnormalities and patients with early relapse after CIT but without adverse cytogenetics. In both groups, 5-year survival probability has increased from <40% in the pre-PI era to >80% with ibrutinib.1,10,90,91
In contrast, the prognosis of R/R patients treated with a first PI after CIT failure remains relatively poor if they harbor TP53 abnormalities. These patients can expect 2-year PFS probabilities of <60%, with the perspective of being rescued with alternate PI. However, it has to be kept in mind that in R/R patients, the majority of early disease progressions on PI (ie, within the first 2 years) occur as RT with usually rapidly fatal outcome.17,50,58 Currently, there are no robust markers for predicting the RT risk on PI.92 In contrast, CLL progression upon PI discontinuation because of toxicity can often be successfully managed by retreatment or PI switching.17,58
The situation worsens once R/R patients become resistant to their first PI, even if their disease remains untransformed. Patients failing idelalisib may respond to ibrutinib, with venetoclax being an additional rescue option. If the first PI has been ibrutinib, however, idelalisib is associated with a high risk of failure and therefore might be discouraged in favor of more effective treatments.11 On the other hand, BTKi-resistant patients have a 60% to 70% chance of responding to venetoclax with a median duration of 2 to 3 years,47-49 but without an established pharmaceutical rescue option once response to venetoclax is lost. If venetoclax is unavailable, idelalisib remains an option for BTKi-resistant patients (supplemental Table 3).
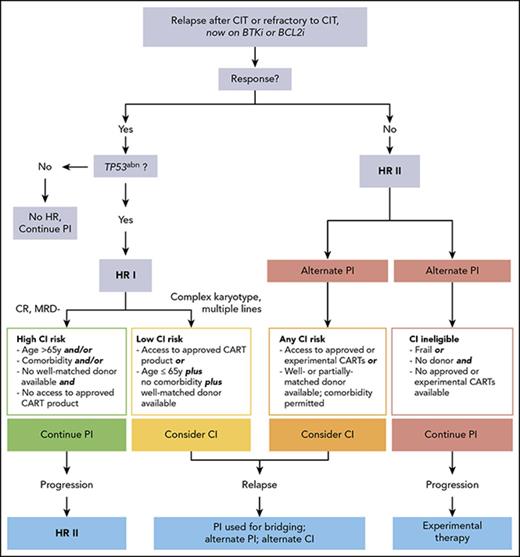
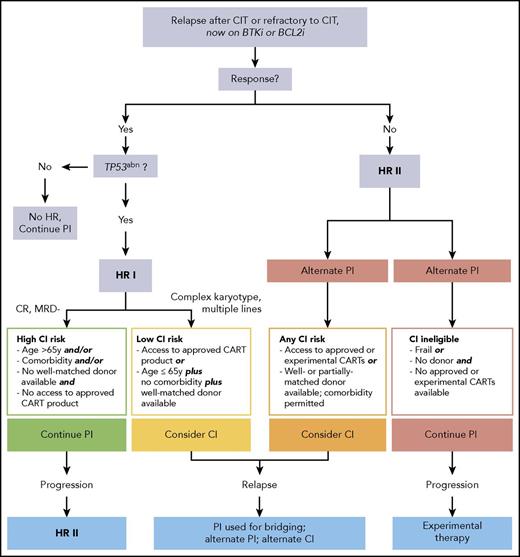
In conclusion, based on the current knowledge on the effectiveness of PIs, high-risk CLL may be redefined by considering (1) the expected duration of response to the PI currently applied and (2) the salvage options remaining once this PI fails (Figure 1). As a result, TN patients with TP53 abnormalities responding to a PI given as first-line therapy as well as R/R patients without unfavorable genetics who are sensitive to a first PI should no longer be considered as high-risk CLL. Hence, 2 high-risk categories can be defined as the following.
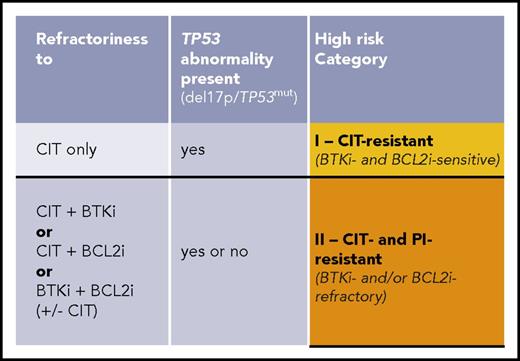
CLL, high-risk-I, CIT-resistant: This category comprises patients with TP53 abnormalities having failed CIT but responding to a first PI (BCRi or BCL2i).
CLL, high-risk-II, CIT- and PI-resistant: This category includes patients who, independent of TP53 status, have failed both CIT and a first PI (BTKi or BCL2i) even if responding to alternate PI. (There is not yet robust information permitting inclusion of patients with TP53 abnormalities resistant to front-line BTKi in this category.)
Supplemental Table 4 shows how the old high-risk criteria translate into the new high-risk CLL concept.
Although patients undergoing disease transformation have a very poor outlook and thus may be considered as having high-risk CLL, RT requires a different therapeutic approach in which PI play only a limited role. Therefore the following considerations are not applicable for transformed disease.
Integration of molecular and cellular therapies and conclusions for a risk-adapted treatment algorithm
PI should be considered as today’s standard of care for patients with high-risk CLL in need for treatment. In the absence of randomized head-to-head comparisons of different PI classes, the choice of the first PI depends on the availability of different PI, their efficacy, and the patient’s comorbidity and individual risk for AE. In contrast, based on current evidence, genetic risk factors, such as CK in addition to TP53 abnormalities, should not be taken into account when selecting the first PI (BTKi or BCL2i). Once maximum response is achieved, there are 2 options to consider: either to continue on PI until progression or intolerance, or to move onto CI. To weigh these options against each other, both alternatives have to be compared for the following intertwined risks and chances: (1) risk of NRM; (2) morbidity risks; (3) risk of progressing in a way precluding successful further treatment (ie, transformation, multiresistance, disease progression with overwhelming deterioration of PS); and (4) chance of long-term disease control and survival.
In the “high-risk-I, CIT-resistant” category, the long-term benefits of moving to CI have to be individually balanced with its morbidity and mortality risks. Regarding alloHCT, a low HCT-specific risk because of younger age (≤65 years) along with absence of comorbidity93 and availability of a well-matched donor94 may argue for moving to transplant. In contrast, a higher HCT-specific risk (older age, relevant comorbidity, or unavailability of a well-matched donor) may favor continuing PI therapy. Moreover, less robust disease-specific risk factors occurring in addition to TP53 abnormalities, such as multiple lines of pretreatment, CK, near-tetraploidy as a risk factor for RT,92 clonal evolution involving driver mutations heralding PI resistance,15,17-19,53 and “accelerated” or “transforming” CLL (but not fulfilling RT criteria),95 may be also considered in decision making. On the other hand, in patients with high-risk-I who achieve MRD clearance and/or CR on venetoclax as a first PI, it seems adequate to postpone HCT. CAR T cells appear to be justified in high-risk-I CLL only if an approved product becomes available.
In the “high-risk-II, CIT- and PI-resistant” category, the risk of fatal progression associated with (secondary) PI continuation increases because rescue options are limited. This may justify considering CI more actively in eligible patients (ie, alloHCT even in case of a higher transplant risk, and CAR T cells even if available only in trials) (Figure 2). The choice of CI (alloHCT vs CAR T cells) will depend on individual factors, such as disease status, donor situation, availability of appropriate CAR T-cell products, and of course patient’s preference.
Decision tree for therapy of chemoimmunotherapy-resistant untransformed CLL according to the revised high-risk concept. *Additional factors to be taken into account when considering cellular therapy. HR, high risk; TP53abn, TP53 abnormality.
Decision tree for therapy of chemoimmunotherapy-resistant untransformed CLL according to the revised high-risk concept. *Additional factors to be taken into account when considering cellular therapy. HR, high risk; TP53abn, TP53 abnormality.
This new risk categorization would refine recent recommendations by the American Society for Blood and Marrow Transplantation, which advises alloHCT in patients with TP53-aberrated R/R CLL responsive or unresponsive to PI with similar strength.96
Although roughly one-half of the patients from the PI studies summarized in supplemental Table 1 were <65 years and thus at “transplantable” age, many of them carrying high-risk genetics, the fraction of patients switched to CI was generally small (<5%), suggesting that cellular therapy may be underused. Reasons for this could be transplant eligibility restrictions, limited availability of CAR T-cell trials, the perception that progression on PI could be rescued without risk by the “next” PI or experimental therapy, and also the misconception that HCT is a dead-end street without rescue options in case of relapse. In reality, development of PI resistance will be fatal for a relevant proportion of patients at each line of therapy because of multiresistance, critical deterioration of PS, or disease transformation.52 On the other hand, untransformed CLL relapsing after alloHCT may remain sensitive to the PI used for bridging to transplant, alternate PI, or alternate CI (ie, donor lymphocyte infusions, CAR T cells after alloHCT failure, or vice versa). Along with the bridging benefit provided by pre-CI PI,3,81,88 this illustrates that PI and CI strategies may be used synergistically for maximizing the outlook of patients with high-risk CLL. In conclusion, molecular and cellular therapies should be considered as complementary rather than competitive therapeutic tools.
Generally, counseling of patients with high-risk CLL as defined here remains challenging given the absence of controlled trials and the still-limited knowledge about the outcome of PI failure. The window of opportunity for sustained disease eradication by CI may be narrow. Future progress in deciphering CLL biology and the identification of critical, and actionable, molecular pathways will hopefully further advance the concepts of high-risk CLL and its management. Meanwhile, the algorithm proposed here should be useful for defining high-risk patient populations for clinical trials and also for their management in daily practice.
The online version of this article contains a data supplement.
Authorship
Contribution: P.D., P.G., J.S., and E.M. designed the concept and wrote the manuscript; and all other authors contributed to further development of the concept, helped write the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: P.D. is a board member of the German Working Group on Marrow and Blood Transplantation (DAG-KBT); he has received honoraria for consultancy for AbbVie, Roche, and Janssen; consultancy and speakers' bureau for Gilead; and served on the speakers' bureau for Kite Pharma. P.G. is the president of the European Research Initiative on CLL (ERIC) and he has received honoraria and research funding from Janssen, Gilead, and AbbVie. J.S. has served on consultancy and speakers' bureaus for AbbVie, Gilead, Janssen, Roche, and Sanofi; and has research funding from Genzyme, Sanofi, GSK, Novartis, and AbbVie. M.M. has served on consultancy and speakers' bureaus for Sanofi, MSD, Octapharma, Pfizer, MAATPharma, and Novartis. M.v.G. has provided consultancy for Gilead and Janssen; served on speakers' bureaus for AbbVie, Gilead, Janssen, and Roche; and received educational support from Gilead. E.K. has received research support from Pfizer and provided consultancy and educational lectures for AbbVie, Celgene, Gilead, Janssen, and Roche. S.S. has received honoraria for advisory boards, consultancy, and speakers' bureaus, and research grants and travel support from AbbVie, AstraZeneca, Celgene, Gilead, Janssen, Mundipharma, and Novartis. E.M. is a board member of ERIC and has received honoraria for consultancy from Janssen, Pharmacyclics, and Morphosys. The remaining authors declare no competing financial interests.
Complete lists of the members of the European Society for Blood and Marrow Transplantation and the European Research Initiative on CLL can be found at https://www.ebmt.org and http://www.ericll.org, respectively.
Correspondence: Peter Dreger, Department of Medicine V, University of Heidelberg, INF 410, 69120 Heidelberg, Germany; e-mail: peter.dreger@med.uni-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal