Abstract
Background: Severe trauma and hemorrhage leads to an acute coagulopathy as exemplified by a decrease in clotting firmness and platelet aggregation. Because platelets contribute 70-80% of clot strength, evaluating the intracellular mechanisms that regulate aggregation in platelets may lead to strategies to mitigate the development of coagulopathy. It is known that platelet aggregation can be inhibited by a rise in intracellular cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP). Changes in cAMP/cGMP would necessitate a change in activity of those enzymes that either synthesize (adenylate cyclase/ guanylate cyclase) or breakdown (phosphodiesterases) these 2nd messengers.
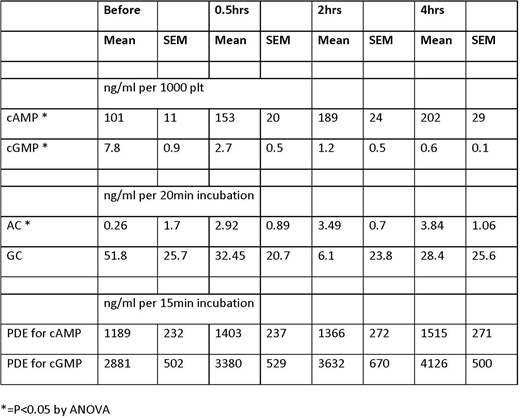
Objective: Determine if the intracellular levels of cAMP and cGMP in platelets rise in rats subjected to polytrauma and hemorrhage. And if so, if there is a change in cyclase or phosphodiesterase activity.
Methods: Polytrauma was induced in isoflurane anesthetized Sprague-Dawley rats (n=10 per group) by damage to the small intestines, right and medial lobes of the liver, the right leg skeletal muscle, and by fracturing the right femur. 40% hemorrhage was then performed immediately after trauma. Rats were euthanized at 4hrs. Platelet intracellular intermediates were measured in whole blood before, and 0.5, 1, 2 and 4hrs after trauma. Blood samples were taken before, and 0.5, 2 and 4 hours after trauma. Platelet rich plasma (PRP) was generated by centrifugation of whole blood at 200g for 10min, no brakes. Cyclic AMP, cGMP, AMP and GMP were extracted from 100ul of PRP after adding 1ml of EtOH, 10mM ammonium formate, with 10ug/ml cGMP-Br as an internal control. IP3 and IP1 (metabolic breakdown product of IP3) were extracted from another 100ul PRP by addition of 50% EtOH, 500mM Formic Acid with 10ug/ml ATP-C13 as an internal control. Samples were centrifuged at 20K g for 10min, and supernatant dried. All Samples were brought up in 200ul of 0.1% formic acid for analysis by LC-MS/MS. Cyclase and phosphodiesterases were isolated from 100ul of rat PRP after sonication to lyse cells, centrifugation to release intracellular constituents, and Spin Column (Zeba, ThermoFisher) removal of small molecular weight constituents. Cyclase activity was measured for 15min after addition of either ATP or GTP and measuring cAMP or cGMP with inhibition of PDE activity (IBMX 1uM). Phosphodiesterase was measured by addition of cAMP or cGMP and measuring AMP or GMP. Data was generated by subtracting measurement from control.
Results: Trauma and hemorrhage led to a significant rise in the intracellular cAMP and a fall in cGMP. Adenylate cyclase activity in platelets also significantly increase after trauma. However, trauma had no effect on guanylate cyclase activity. Phosphodiesterase activity was elevated after trauma for both conversion of cAMP and cGMP to AMP and GMP, but neither was significant.
Conclusion: Trauma and hemorrhage leads to coagulopathy and platelet dysfunction. The platelet dysfunction is likely due to a rise in the intracellular cAMP, but not to cGMP as it fell precipitously after trauma. The rise in cAMP is likely due to an increase in adenylate cyclase activity induced by trauma. Although there was a rise in PDE activity, it was not significant, but may play a role in blunting the rise and cAMP, and contribute to the fall in cGMP. This study was funded by the US Army MRMC and conducted in compliance with the Animal Welfare Act, the implementing of Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal