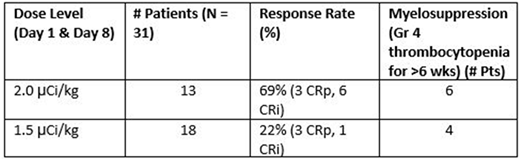
Abstract
Background: Older patients (pts) with AML unfit for intense induction chemotherapy have a poor prognosis with a 5 year survival of <10%. 225Ac-lintuzumab is composed of 225Ac linked to a humanized anti-CD33 monoclonal antibody. Data were previously presented on the initial 13 pts who received 2.0 µCi/kg/dose on Days 1 and 8 (ASH, 2017, Abstract 616). Although that dose resulted in a high response rate of 69%, there were 6 pts with Grade 4 thrombocytopenia lasting >6 weeks and 5 CRi patients did not reach an ANC of ≥ 500/µL. Therefore, the activity level was reduced to 1.5 µCi/kg/dose for further evaluation.
Methods: This study enrolled older pts with untreated AML who were considered to be unfit for standard induction chemotherapy. Pts aged 60 to 74 years were required to have significant co-morbidities, while all pts ≥ 75 years were eligible. Other eligibility criteria included ECOG PS 0-2, CD33 expression on > 25% of blasts, and a peripheral blast count <200/µL within 10 days of the first dose to optimize targeting of bone marrow (BM) blasts. Hydroxyurea was used to lower peripheral blast counts. 1.5 µCi/kg of 225Ac-lin were administered IV on Days 1 and 8. G-CSF was given starting 10 days after the 2nd dose and spironolactone was given for up to 1 year to minimize the risk of radiation-induced nephrotoxicity. Pts were evaluated for the CD33 splicing polymorphism SNP rs12459419.
Results: 18 pts were included in this prespecified Interim Analysis. An additional 9 pts were treated at this dose and will be included in an updated analysis at the ASH meeting. The median age of the 18 pts was 73.5 years (range 60-87) and median ECOG Performance Status was 1 (0 in 3 pts, 1 in 9 pts, & 6 in 2 pts). 11 pts had prior AHDs (6 MDS, 2 CMML, 2 NHL, 1 myelofibrosis), and 9 had prior treatment for AHDs. Of the pts with known cytogenetic and molecular genetic results, 1 had favorable-risk, 3 had intermediate-risk, and 5 had adverse-risk AML using NCCN guidelines. The median baseline BM blast % was 40.5% (range, 22-66%) with a median CD33 expression of 62% (range, 26-100%) of AML cells.
Objective responses were seen in 4 pts (22%): 3 complete remissions with incomplete platelet count recovery (CRp) and 1 complete remission with incomplete hematologic recovery (CRi). Among the responders with known cytogenetics, 1 pt had adverse genetics and 1 had Intermediate-risk genetics.Of the pts with remission, 3 had CT genotype for the CD33 splicing polymorphism SNP rs12459419 (including 2 CRp) and 1 had CC genotype. 3 of the responders are in follow up at Days 59, 169 and 266 without further treatment.
Myelosuppression was seen in all pts including Grade 4 thrombocytopenia with marrow aplasia for > 6 weeks after the first dose in 4 pts. 1 pt with prior MDS had pancytopenia for > 4 months. The 3 pts with CRp achieved an ANC ≥ 1000/µL at Days 28, 38, and 40 from the first dose of 225Ac-lin. The pt with CRi did not reach an ANC of 500/µL.
Non hematologic Grade ≥3 Treatment-Emergent adverse events (AEs) that were at least possibly related were 4 patients with febrile neutropenia, as well as 1 patient each with fungal pneumonia, acute respiratory failure, pulmonary edema, chest pain, sepsis, gastric hemorrhage, generalized muscle weakness, atrial fibrillation with rapid ventricular response and typhilitis. No pts had an infusion-related reaction requiring dose interruption. Veno-occlusive disease did not occur. The 30-day mortality rate was 16.7% (1 cardiac arrest and 2 with multi organ failure).
Conclusions: Preliminary data from this Interim Analysis of 225Ac-lin monotherapy in older AML pts unfit for intensive therapy indicate a lower rate of myelosuppression at 1.5 µCi/kg/dose but also a lower response rate than was seen at 2.0 µCi/kg/dose. Although the study met the prespecified response criteria for continuing enrollment, it will be closed to further accrual in recognition that targeted radiation, like other therapies, will likely have the best outcomes when used in combination with other therapies in pts with active AML. An extensive development program in AML, MDS, and multiple myeloma is planned. One study will utilize 225Ac-lin in combination with salvage chemotherapy and another with venetoclax. 225Ac-lin will be used as a single agent for AML postremission therapy. In addition, 225Ac-lin will be used as targeted conditioning prior to hematopoietic stem cell transplant in pts with MDS with a complex karyotype and as a conditioning agent prior to autologous transplant in MM.
Atallah:Jazz: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Abbvie: Consultancy; BMS: Consultancy. Orozco:Actinium Pharmaceuticals: Research Funding. Craig:Novartis: Research Funding; Actinium Pharmaceuticals: Research Funding; Celgene: Research Funding. Levy:Takeda (Millennium Pharmaceuticals, Inc.): Consultancy. Finn:Ochsner Clinic Foundation: Employment. Perl:NewLink Genetics: Membership on an entity's Board of Directors or advisory committees; Actinium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy; Astellas: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees. Park:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Consultancy, Research Funding; Shire: Consultancy; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Kite Pharma: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. Roboz:Astex Pharmaceuticals: Consultancy; Bayer: Consultancy; Aphivena Therapeutics: Consultancy; Celltrion: Consultancy; Celgene Corporation: Consultancy; Novartis: Consultancy; Celgene Corporation: Consultancy; Pfizer: Consultancy; Argenx: Consultancy; Roche/Genentech: Consultancy; AbbVie: Consultancy; Cellectis: Research Funding; Aphivena Therapeutics: Consultancy; Roche/Genentech: Consultancy; Sandoz: Consultancy; Bayer: Consultancy; Sandoz: Consultancy; Jazz Pharmaceuticals: Consultancy; Otsuka: Consultancy; Astex Pharmaceuticals: Consultancy; Daiichi Sankyo: Consultancy; Orsenix: Consultancy; Janssen Pharmaceuticals: Consultancy; AbbVie: Consultancy; Jazz Pharmaceuticals: Consultancy; Argenx: Consultancy; Janssen Pharmaceuticals: Consultancy; Eisai: Consultancy; Otsuka: Consultancy; Pfizer: Consultancy; Eisai: Consultancy; Orsenix: Consultancy; Cellectis: Research Funding; Daiichi Sankyo: Consultancy; Novartis: Consultancy; Celltrion: Consultancy. Tse:Brown Cancer Center, University of Louisville School of Medicine: Employment; Jazz: Consultancy; Amgen: Consultancy; Amgen: Honoraria; Amgen: Honoraria. Mawad:Swedish Cancer Institute: Employment. Rizzieri:Novartis: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Teva: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Arog: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Berger:Actinium Pharmaceuticals: Employment, Equity Ownership. Jurcic:Astellas: Research Funding; Daiichi-Sankyo: Research Funding; Incyte: Consultancy; Celgene: Research Funding; AbbVie: Consultancy, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics: Research Funding; Syros Pharmaceuticals: Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Genetech: Research Funding; Kura Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal