Abstract
Introduction: Peripheral T-cell lymphomas (PTCL) are a rare heterogeneous group of lymphoid malignancies, which originate from post-thymic (or mature) T-cells. Although there are approximately 7,000 new cases of PTCL in the US annually, PTCL is extremely heterogenous and difficult to diagnose even in academic settings (Teras et al. 2016. CA Cancer J Clin 2016; 66:443-459). Prior research suggests a high rate of diagnostic revision among PTCL patients. The objective of this analysis was to describe the rate of diagnostic revision among patients with a PTCL diagnosis using 2 large, real-world US databases.
Methods: This retrospective analysis used the IBM MarketScan Commercial and Medicare Supplemental administrative claims databases (claims) and the IBM Explorys Research Database, an electronic health record (EHR) database; both databases have national coverage but are fundamentally different in their structure and data sources. Adults (age ≥18 years) with a new diagnosis of PTCL between January 2010 and June 2017 were identified in each database. The prevalence of diagnostic revision was the primary outcome, defined as the diagnosis of a non-PTCL diagnosis before or after the PTCL diagnosis with the latest diagnosis serving as the index date. Other lymphomas included were Hodgkin's, diffuse large-B-cell (DLBCL) and marginal zone lymphoma. The presence of anaplastic large cell lymphoma, mature T/NK-cell lymphomas, and other subtypes of T/NK-cell lymphomas closely related to PTCL were described but were not classified as diagnostic revision. Patients were required to have 12 months of pre-index and one month of post-index continuous enrollment (claims) or evidence of database activity (EHR).
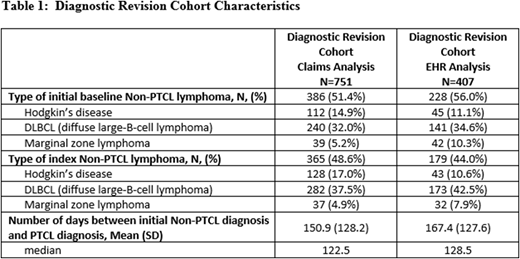
Results: 2561 patients met the selection criteria for the claims database analysis (mean age 59.9 [SD=15.4]; 42.9% female) and 1,881 met the EHR criteria (mean age 64.3 [SD=15.2]; 47.0% female). Diagnostic revision was present among 29.3% of PTCL patients in the claims analysis and 21.6% of PTCL patients in the EHR analysis. Among patients with diagnostic revision, 51.4% revised to PTCL and 48.6% revised from PTCL in the claims analysis, while 56.0% revised to PTCL and 44.0% revised away from PTCL in the EHR analysis. Among patients with diagnostic revision from PTCL, 37.5% were revised to DLBCL, 17.0% revised to Hodgkin's disease, and 4.9% to marginal zone lymphoma in the claims analysis, and 42.5% revised to DLBCL, 10.6% revised to Hodgkin's disease, and 7.9% to marginal zone lymphoma in the EHR analysis. Among patients with diagnostic revision to PTCL, 32.0% revised from DLBCL, 14.9% revised from Hodgkin's disease, and 5.2% from marginal zone lymphoma in the claims analysis, and 34.6% revised from DLBCL, 11.1% revised from Hodgkin's disease, and 10.3% from marginal zone lymphoma in the EHR analysis. The average time from the first diagnosis to the final diagnosis in the 12-month baseline period was 150.9 [SD=128.2; median 122.5] days in the claims analysis and 167.4 [SD=127.6; median=128.5] days in the EHR analysis (Table 1). Baseline Deyo Charlson Comorbidity Index scores were higher among patients with diagnostic revision in both the claims (4.1 [SD=2.8] vs. 2.8 [SD=2.5]) and EHR (2.4 [SD=2.5] vs. 4.4 [SD=3.1]) analysis (both p<0.01), suggesting clinical differences between these cohorts. Average post-index follow-up was 668.1 [SD=577.0] days for the claims and 852.8 [SD=676.2] for the EHR analyses.
Conclusions: This analysis reaffirms the need for appropriate diagnostic criteria and expertise when diagnosing PTCL and its subtypes. The analysis is limited to patients who meet the inclusion criteria for 1 of the 2 databases and generalizability of study results are subject to those same limitations. Differences in diagnostic revision rates between the databases may be due to underlying differences in patient populations in the data sources or potential coding differences in data contributors. Diagnostic revision is common among subtypes of PTCL and can serve as area for quality improvement in the field of hematology oncology to promote accurate diagnosis closer to when lymphoma presentation becomes apparent.
Feliciano:Seattle Genetics: Employment, Equity Ownership. Bonafede:Seattle Genetics: Other: I am an employee of IBM Watson Health (formerly Truven Health Analytics), which received a research contract to conduct this study with and on behalf of Seattle Genetics.. McMorrow:Seattle Genetics: Other: I am an employee of IBM Watson Health (formerly Truven Health Analytics), which received a research contract to conduct this study with and on behalf of Seattle Genetics.. Park:Seattle Genetics: Other: I am an employee of IBM Watson Health (formerly Truven Health Analytics), which received a research contract to conduct this study with and on behalf of Seattle Genetics.. Lisano:Seattle Genetics: Employment, Equity Ownership. Manley:Seattle Genetics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal