Abstract
BACKGROUND: ABVD with or without radiation is standard therapy for limited stage HL, but carries risks of bleomycin-lung injury and radiation toxicity. Brentuximab vedotin (BV) is an anti-CD30 antibody drug conjugate which is highly active in relapsed classical HL. We previously combined BV with AVD in limited stage non-bulky classical HL which resulted in a high CR rate, but at the cost of increased neutropenia, neutropenic fever, and neuropathy, likely related to the overlapping toxicity profile with vinblastine. Similar toxicity findings were also observed with BV-AVD in advanced stage classical Hodgkin lymphoma. We therefore evaluated BV plus AD (BV-AD) without radiation therapy for non-bulky stage I-II classical HL with the goal of reducing toxicity and maintaining high rates of inducing CR.
METHODS: This is a multicenter single arm open label phase 2 study. Patients received BV 1.2 mg/kg plus standard dose adriamycin and dacarbazine on days 1 and 15 of each 28 day cycle. GCSF prophylaxis was not included. Patients received 4 or 6 cycles of BV-AD based on the results of an interim PETCT scan performed following cycle 2. PET negativity was defined as Deauville scores 1-3. Patients in CR on interim PETCT received 4 total cycles of therapy; patients in PR completed 6 cycles. The primary endpoint is complete response rate (CRR) at end of treatment. A sample size of 34 was required to detect an end of treatment CRR of 95% with 91% power and alpha error of 0.09.
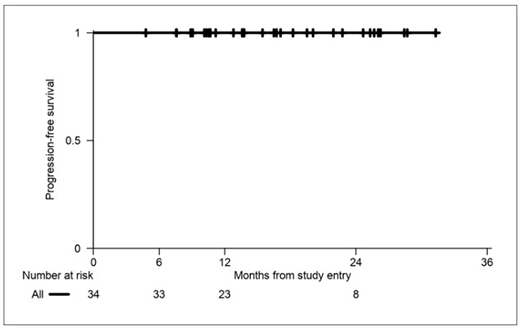
RESULTS: 34 patients were enrolled. Median age is 36 (range 18-63). Stage is IA (3), IB (1), IIA (29) and IIB (1). Risk is classified per the GHSG criteria as early unfavorable in 47%, and favorable in 53%. The interim CR rate is 94%. Accordingly, 32 interim PET negative patients (94%) received 4 total cycles of therapy, and 2 interim PET positive patients (6%) received 6 total cycles of therapy. No patients received consolidative radiation therapy, per protocol. The primary endpoint of end of treatment CR rate is 100%. At a median follow-up of 15 months, the FFS, PFS and OS are all 100%. The most common adverse events of any grade are nausea (79%), peripheral sensory neuropathy (56%), fatigue (50%), constipation (38%), alopecia (35%) and neutropenia (24%). Most toxicities were low grade, with only 15% of subjects experiencing any grade 3 toxicity, and there were no grade 4 or 5 toxicities. Specifically, 2 patients had grade 3 neutropenia, and 1 patient each had grade 3 nausea/vomiting, pneumonia and thromboembolic event. Peripheral sensory neuropathy was grade 1 in 17 patients, and grade 2 in 2 patients. There were no cases of neutropenic fever.
CONCLUSIONS: BV-AD for 4-6 cycles induces high interim and end of treatment CR rates of 94% and 100%, respectively, allowing 4 total cycles of therapy in most patients. The PFS, FFS and OS are all 100% at last follow up. Toxicity appears mild and notable for a low incidence of neutropenia, alopecia, and moderate peripheral neuropathy. This promising regimen avoids bleomycin, vinblastine, radiation and primary GCSF prophylaxis with resultant low toxicity and preserved high efficacy rates in patients with early favorable and early unfavorable non-bulky limited stage classical Hodgkin lymphoma. Follow up for this trial is ongoing.
Abramson:Merck: Consultancy; Seattle Genetics: Consultancy; Karyopharm: Consultancy; Verastem: Consultancy; Amgen: Consultancy; Humanigen: Consultancy; Juno Therapeutics: Consultancy; Gilead: Consultancy; Novartis: Consultancy; Bayer: Consultancy; Celgene: Consultancy. Sokol:Mallinckrodt Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy; Spectrum Pharmaceuticals: Consultancy. Jacobsen:Merck: Consultancy; Seattle Genetics: Consultancy. LaCasce:Seattle Genetics: Consultancy, Honoraria; Research to Practice: Speakers Bureau; Humanigen: Consultancy, Honoraria; Bristol-Myers Squibb: Other: Data safety and monitoring board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal