Abstract
BACKGROUND: Chimeric antigen receptor (CAR) T cells have shown enormous promise in the treatment of certain B cell malignancies. Access to treatment is still limited due to a variety of issues, including pricing and centralized manufacturing models. Generation of CAR-T cells using an automated platform, followed by rigorous functional phenotyping, may contribute to the development of a robust long-lasting therapy.
METHODS: Here, we used the Miltenyi Prodigy (Miltenyi Biotech, Bergisch Gladbach, Germany) to automate the process of manufacturing genetically manipulated T cells in a closed system. The system obviates the need for clean room infrastructure. We tested the feasibility of utilizing the Miltenyi Prodigy to manufacture CAR-T cells using a CD19 scFV vector with the 4-1BB co-stimulatory domain. (Lentigen Technology, Inc, Gaithersburg, MD). The purity, differentiation capacity and effector function of the enriched CAR-T cells was studied using high-dimensional flow cytometry. Finally, the functional potential of these cells was tested in vitro and by treating NOD-SCID-gamma (NSG) mice infused with B cell lymphoma cells (Raji B cell), with the CAR-T cells.
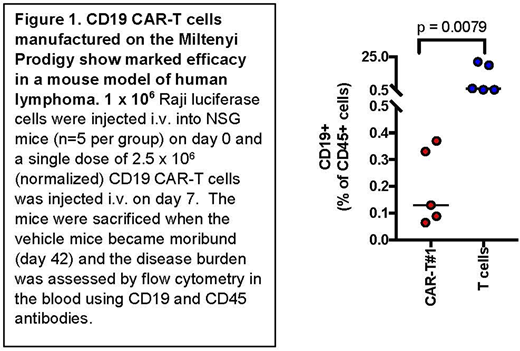
RESULTS: Starting with 1 x 108 CD4 and CD8 cells from donor apheresis products, CAR-T cells were expanded for 12 days in culture media containing with TransAct (Miltenyi Biotech), IL7 and IL15. The mean fold-expansion at day 12 was 44 ± 5.6, range 39-50 (n=3). The mean transduction efficiency of CAR-T vector was 20%, range 10-25% (n=3), which is similar to other reported methods. The CD19 CAR-T product was enriched in both the CD4 and CD8 T cells subsets, and showed high-level of cytotoxicity against CD19+ cell lines in vitro and in vivo (Figure 1: Mice treated with the CD19-CAR T demonstrated a marked reduction in disease burden as compared to T cell control as measured by bioluminescence imaging and flow cytometric analysis). The CAR-T product was enriched in cell subsets with both effector (CD27-CCR7-; ~20% of total cells) and central memory phenotypes (CD27+CCR7+; ~30% of total T cells). The effector CD4 and CD8 T cells showed increased expression of major functional T cell differentiation transcription factors (i.e. T-bet and GATA3) critical for the development of anti-tumor responses. Whereas, the central CD4 and CD8 T cells were enriched for the expression of TCF7 (a stemness related member of the WNT signaling known to increase longevity of these cells). The frequencies and phenotypes of these cells were maintained in peripheral blood of NSG mice infused with B cell lymphoma cells (Raji B cells), 1 week after treatment. A significant expansion of CD8+ effector T cells and a dramatic reduction in tumor burden was observed over the next 4 weeks in all major organs. Interestingly, we observed that smaller proportion of central-memory like cells (with higher TCF7 levels) continued to persist 6 weeks post-treatment, potentially contributing to a long-lived recallable response. Based on these data we have initiated a phase 1 clinical trial to test the therapeutic potential of the CAR-T product in patients with advanced/refractory B cell lymphoma. The first clinical grade manufacturing run resulted in a CD19 + cell yield of 1.4 x109.
CONCLUSION: Our data highlight that the automated CAR-T generation platform (i.e. Miltenyi Prodigy) is effective at the generating purified functionally competent CAR-T cells. Further, findings from our phenotyping analyses show that the CAR-T product is enriched in both effector and central memory subsets and is effective at tumor clearance in vivo. Thus far, we have treated one patient with CD19 CAR-T manufactured on this platform and 2 more have been enrolled. Though this initial study is based on CD19 CAR-T cells, the approach described here could easily be utilized to genetically engineer T cells with gene constructs that are more relevant for specific cancers and infectious diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal