Abstract
Background:
Since the US Food and Drug Administration (FDA) approved the first BCR-ABL1-targeted tyrosine kinase inhibitor (TKI) imatinib in 2002 as the first line therapy1, chronic phase chronic myeloid leukemia (CML-CP), once a uniformly fatal disease, now has become a disease that is controlled in >90% patients2. Conventional treatment for CML before TKIs included hydroxyurea, interferon alpha with or without cytarabine, busulfan, as well as stem cell transplantation3. Currently, there are five TKIs approved by FDA for chronic-phase CML treatment: imatinib, nilotinib, dasatinib, bosutinib, and ponatinib4.Studies have suggested that CML patients have higher baseline cardiovascular comorbidities than general US population5. Moreover, TKIs, especially later generation, are associated with cardiovascular toxicities including myocardial infarction, ischemic heart disease, cerebrovascular disease, peripheral arterial disease, QT prolongation, hypertension, and venous thrombosis1,4,6-9. As patients may need life-long (or minimum of several years') therapy, the cardiovascular safety profile of TKI treatment warrants utmost attention. Thus, we examined the effect of TKIs on cardiovascular mortality using the SEER database.
Methods:
Data were extracted from the Surveillance, Epidemiology and End Results (SEER) 18 program 1992-2004. Cardiovascular diseases were defined as diseases of the heart, hypertension, cerebrovascular disease, atherosclerosis, aortic aneurysm and dissection, and other disease of arteries, arterioles, and capillaries. Time of diagnosis between 1992-2002 was defined as pre-TKI era and 2003-2014 as TKI era. Patients had BCR/ABL negative CML or any other cancer before CML were excluded.
T-test and chi-square test were used to analyze the baseline characteristics. Multivariable Cox proportional hazards regression was used to calculate the hazard ratio (HR). Sub-distribution hazard ratios (SHRs) for cardiovascular disease (CVD) specific mortality were calculated by using Fine and Gray competing-risks model. All above statistical tests were performed assuming a 2-sided alpha of 0.05 by using SAS 9.4. CVD standard mortality ratio (SMR) and absolute excess risk (AER) were calculated by using SEER*Stat 8.3.5 software MP-SIR Matrix comparing to the total U.S. population (1969-2016 Counties).
Results:
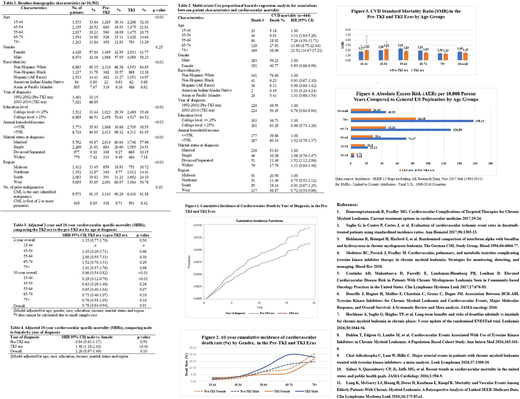
A total of 10,502 patients were included (Table 1). Table 2 shows that as expected, increased age was associated with higher CVD mortality. Females had a 20% decreased risk of overall CVD mortality compared to males. Notably, those diagnosed in the TKI era had better cardiovascular outcome than the pre-TKI diagnosis, HR=0.78 (95%CI: 0.64-0.94), which was also illustrated in Figure 1. In the adjusted model (Table 3), the significantly decreased mortality in the TKI era was mostly observed at 10-year follow up and among the youngest age group 15-44 years. The 10-year cardiovascular mortality also differed by gender, especially after age 54 (Fig. 2). In the TKI era, males had a 58% increased 10-year CVD mortality (p<0.01) compared to females (Table 4). In comparison to US general population (Fig. 3), CML patients had higher cardiovascular mortality, regardless of year of diagnosis. However, the overall CVD was higher in the pre-TKI era: 1.69 (95% CI: 1.51-1.89) vs. 1.45 (95%CI: 1.24-1.69) in the TKI era. Additionally, the AERs in the TKI era across all age groups were significantly lower compared to the pre-TKI era (Figure 4).
Conclusions:
Though TKIs appear to be associated with increased cardiovascular toxicity, we herein report an observed decreased cardiovascular mortality among CML patients diagnosed in the TKI era relative to the pre-TKI era. One potential explanation is that TKIs might be less cardiotoxic than conventional chemotherapies such as busulfan, cytarabine, or interferon; additionally these findings could be due to overall decreased CVD mortality since 200010. While CVD mortality among CML patients improved after 2002, it was still higher than the general US population. Besides the cardiovascular toxicities of TKIs, underlying CML associated factors could also be a potential driver for increased CVD mortality11. Our results argue for aggressive management, in general, of CVD risk factors among CML patients, especially elderly and males, and further investigation into specific mechanisms, factors and predictors of risk in TKI-treated CML.
Mauro:Takeda: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal