Abstract
Background
The B-cell receptor (BCR) signaling pathway and the pro-survival Bcl-2-family of proteins play crucial roles in the pathogenesis of chronic lymphocytic leukemia (CLL). Constitutive activity of the BCR signaling pathway and overexpression of Bcl-2 promote CLL-cell survival, proliferation and drug resistance. BCR-targeted therapies, most notably ibrutinib and idelalisib and the Bcl-2 inhibitor Venetoclax, demonstrate the potential of targeting these pathways. However, there is no evidence that these novel agents are curative in the event of relapse. Treatment options remain limited for patients treated with these agents, in particular those with TP53 lesions.
In our recent study (Crassini et al., BJH 2018) we demonstrated efficacy of the PI3/PIM kinase inhibitor, IBL-202 (Inflection Biosciences, Ltd), against CLL cells. In the current study we investigated the effects of combining IBL-202 with Venetoclax on primary CLL cells and both wild-type and TP53 deficient OSU-CLL cells.
Methods
Primary CLL cells were co-cultured with CD40L-expressing fibroblasts. We established a TP53 knock-out OSU-CLL cell line (OSU-TP53ko) using the CRISPr-Cas9 system. Cell viability was assessed using the mitochondrial dye DilC1(5), propidium iodide and flow cytometry. Synergy between IBL-202 and Venetoclax was evaluated by determining combination indices (CI) using the Compusyn software. The effects of the drugs on cell cycle and proliferation of the OSU-CLL cell lines were assessed using propidium iodide or carboxyfluorescein succinimidyl ester (CFSE) and flow cytometry. The effects of the drugs on the migratory capacity of CLL cells were assessed by determining changes in CXCR4 expression and CLL-cell migration along an SDF-1a gradient. The mechanisms of action of the drugs were investigated by immunoblotting.
Results
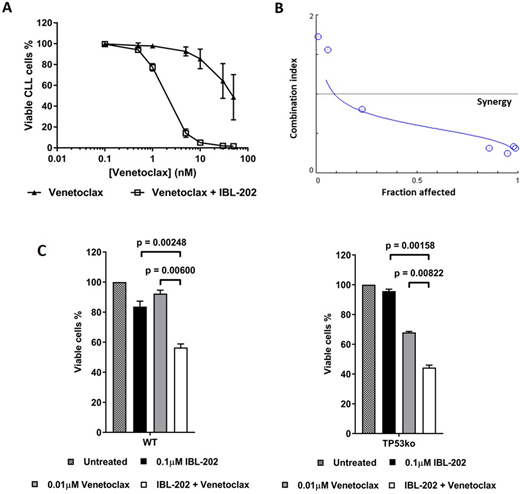
IBL-202 and Venetoclax were highly synergistic against primary CLL cells co-cultured with CD40L-fibroblasts, with a CI of 0.4 at a fractional effect of 0.9 (Figure A and B). Synergy between the drugs was consistent with a significant (P < 0.05) reduction in the IC50 for both drugs.
Synergy was also observed against wild-type (WT) and TP53ko OSU-CLL cells, with CI values of < 0.5 at fractional effects of 0.5 and 0.9. Synergy was consistent with significantly greater cytotoxic effects of the drugs in combination (Figure C. WT : P = 0.002 and TP53ko : P = 0.002). IBL-202 and Venetoclax in combination induced cell cycle arrest and slowed the proliferation of both cell lines.
Immunoblotting of primary CLL cells showed IBL-202, alone and in combination with Venetoclax, inhibited AKT phosphorylation and reduced the expression of Mcl-1 and Bcl-xL.
A greater than additive effect of IBL-202 and Venetoclax was observed on the migratory capacity of CLL cells, reducing the number of cells migrating towards SDF1-a. The effects of the drugs on cell migration were consistent with reduced expression of CXCR4.
Conclusions
The synergy we observed between IBL-202 and venetoclax against primary CLL cells cultured under conditions that mimic the tumor microenvironment suggests this drug combination may be effective against CLL cells within the lymph nodes and bone marrow. Furthermore, the efficacy of the combination against the TP53ko OSU-CLL cell line suggests the combination may be a highly effective treatment strategy for poor risk CLL disease.
Figure A and B - Synergy of IBL-202 and Venetoclax against primary CLL cells.
Figure C - Cytotoxic effects of IBL-202 in combination with Venetoclax against OSU and OSU-TP53ko CLL cells
O'Dwyer:Onkimmune: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Glycomimetics: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal