Abstract
Background:
Autologous stem cell transplantation (ASCT) is the standard of care for eligible patients with newly diagnosed multiple myeloma (MM). Typically, patients receive several cycles of induction therapy to achieve appropriate cytoreduction prior to ASCT. Since the introduction of lenalidomide into induction therapy, there have been conflicting reports about its impact on subsequent autologous peripheral blood stem cell (PBSC) mobilization. Early reports showed a trend of patients failing PBSC mobilization and collection after lenalidomide when granulocyte colony stimulating factor (GCSF) was employed as a single agent. More recent reports suggested that using chemotherapy in combination with growth factors including plerixafor can overcome failures of mobilization. To address these conflicting reports, we evaluated the impact of prior lenalidomide in a large cohort of MM patients undergoing mobilization and collection at a tertiary stem cell transplant center.
Methods:
The primary aim of this study was to identify the impact of prolonged lenalidomide therapy on mobilization of autologous PBSCs. We examined all patients 18 years of age and older, with a confirmed diagnosis of MM who attempted stem cell mobilization and collection between January 2012 and July 2015 at the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Collected information included: demographics, staging according to the International Staging System, results of cytogenetics by conventional karyotype or FISH, prior treatments, number of cycles of lenalidomide received, mobilization method, receipt of plerixafor, number of stem cells collected, days of collection, and receipt of prior radiation therapy. Patients were also scored as successful or failed mobilization (defined as collecting < 2.5 x 106 CD34+ cells/kg). The patients were categorized into 3 groups (based on our recommendations for induction duration ≤ 6 cycles) for analysis: 1) Those patients with prior receipt of > 6 cycles lenalidomide, 2) Those patients with prior receipt of ≤ 6 cycles of lenalidomide, and 3) Patients without prior lenalidomide exposure.
Results:
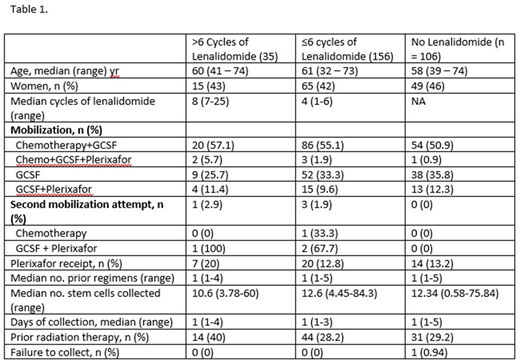
We identified 297 patients with MM who underwent mobilization of PBSCs. Of these, 35 received > 6 cycles of lenalidomide (median of 8 cycles, range of 7 - 25), 156 received ≤ 6 cycles of lenalidomide (median of 4 cycles, range 1 - 6), and 106 received no lenalidomide (Table 1). Similar proportions of patients in each group of lenalidomide exposure received chemotherapy mobilization, chemotherapy with plerixafor, GCSF alone, and GCSF with plerixafor. There was no significant difference in receipt of plerixafor between these groups (p=0.41). A second mobilization attempt was made in 1 patient (2.9%) who received > 6 cycles of lenalidomide, 1.9% of patients who received ≤ 6 cycles of lenalidomide, and 0% of patients with no prior lenalidomide exposure. Only one patient, who had not received lenalidomide previously, failed to collect. In a multivariate linear regression analysis for association between receipt of > 6 cycles of lenalidomide with total number of PBSCs collected, with other covariates including prior radiation therapy, age, and number of prior regimens received, we did not observe a significant effect (regression coefficient of -3.37, p=0.166). We then examined whether receiving > 6 cycles of lenalidomide was associated with days of stem cell collection in a multivariate regression; other covariates included prior radiation therapy, age, mobilization regimen, and number of prior regimens, and did not see any association (regression coefficient -0.053, p=0.697).
Discussion:
In this retrospective analysis of MM patients undergoing autologous PBSC mobilization at a single center, we show that prior lenalidomide exposure did not meaningfully impact the total number of PBSC collected or the number of days of apheresis after controlling for clinically relevant features in a multivariate model. These data suggest that longer courses of induction therapy with lenalidomide-containing regimens to achieve a maximum response before transplant should not adversely impact the ability to collect PBSC, and that arbitrarily limiting lenalidomide use pre-mobilization does not appear warranted. The extensive use of chemotherapy with G-CSF and plerixafor for mobilization at our center may account in part for these findings.
Cowan:Abbvie: Research Funding; Janssen: Research Funding; Juno Therapeutics: Research Funding; Sanofi: Research Funding. Becker:GlycoMimetics: Research Funding. Gopal:Incyte: Consultancy; Aptevo: Consultancy; Janssen: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; BMS: Research Funding; Teva: Research Funding; Brim: Consultancy; Asana: Consultancy; Merck: Research Funding; Pfizer: Research Funding; Spectrum: Research Funding; Takeda: Research Funding. Holmberg:Millenium-Takeda: Research Funding; Sanofi-Genzyme: Research Funding; Seattle Genetics: Research Funding; Merck Sharpe: Research Funding; Jazz Pharmaceuticals: Consultancy; NCCN: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal