Abstract
Introduction
Light chain (AL) amyloidosis is a low burden plasma cell disorder, characterized by deposition of misfolded lambda or kappa light chains. Kidney dysfunction is present in almost two-thirds of patients at the time of initial presentation, followed by diastolic heart failure in about 50% of cases, which is responsible for 75% of deaths in these patients. Autologous stem cell transplant (auto-SCT) remains the gold standard for the management of AL amyloidosis but is often impractical to perform by virtue of patients' age, medical comorbidities including cardiac involvement.
Methods
We conducted a literature search using three databases (PubMed, Embase,Web of Science). Our search strategy included MeSH terms and key words such as AL amyloidosis, daratumumab and darzalex from date of inception to March 2018. After excluding duplicates, reviews and non-relevant articles, we selected eight studies, including two case reports, two phase II prospective trials and four retrospective trials.
Results
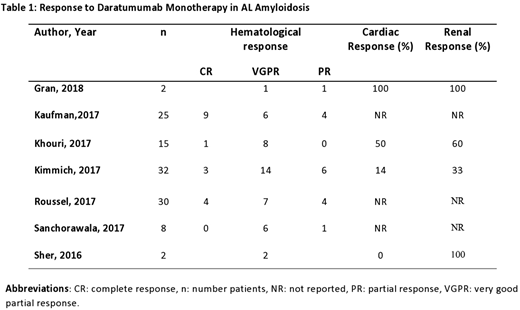
Data on 129 patients was included, there ages ranged from 43-83 years. Median number of prior therapies were 3 (range: 2-6), 106 (82%) received proteasome inhibitor (bortezomib) based therapy, and 69 (53.5%) received immunomodulatory (lenalidomide) based therapy. Another 41 (32%) received high dose melphalan (HDM) followed by auto-SCT. The time from the diagnosis of AL to the start of daratumumab therapy varied from 0.7-150 months. Eighty-nine (69%) patients had cardiac and 64 (49.6%) patients had renal involvement. A total of 114 (88%) patients received a daratumumab dose of 16 mg/kg weekly for 8 weeks followed by every 2 weeks for the next 8 weeks.
A total of 104 patients were evaluable for hematological response, assessed by improvement in free light chain (FLC) levels. Daratumamab achieved an impressive overall response rate (ORR) of 72% (n=75). Complete remission (CR) in 15 (14%) of patients, very good partial response (VGPR) in 44 (42%) and a partial response (PR) in 16 (15%) of patients was noted. Thirty-four patients with cardiac involvement and 26 patients with renal amyloidosis were assessed for organ response across four studies. Thirteen (38%) patients with cardiac amyloidosis demonstrated an improvement in N-terminal pro brain natriuretic peptide (NT-proBNP) levels. Ten (38%) patients with renal involvement responded according to consensus criteria [Palladini et al 2014] for organ response. Another two had improvement in serum creatinine levels.
Among the 129 patients treated with daratumumab for AL amyloidosis, 36 (32%) reported infusion related reactions (IRR). Most were mild (grade 1-2). Daratumumab infusion was well tolerated in patients with cardiac (n=54) and renal involvement (n=48). Only one patient needed adjustment in his diuretic dose, another one developed decompensated heart failure and one died due to progression of cardiac disease. Seven patients had worsening of their NT-proBNP levels. Similarly, no dose adjustments were required for patients with renal amyloidosis and one patient tolerated daratumumab infusion at a GFR<20 mL/min without any complications.
Conclusion
Daratumumab monotherapy is associated with deep and prompt hematological responses in patients with heavily pretreated AL amyloidosis, at the standard dosing regimens used for multiple myeloma, with a favorable safety profile. Furthermore, daratumumab performed well in patients with cardiac amyloidosis even though there is an increased risk of volume overload and infusion related morbidity. Given the high incidence of peripheral neuropathy with bortezomib, cardiotoxicity with carfilzomib based regimens in amyloidosis patients, daratumumab appears to be a suitable alternative. It has already been approved for relapsed amyloidosis (AL) patients in the European Union. Currently, it is being investigated as monotherapy for AL amyloidosis in phase 2 trials (NCT02841033 and NCT02816476) and in combination with bortezomib, cytoxin and dexamethasone (VCd) in a phase III trial (NCT03201965).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal