Abstract
Introduction
Prophylactic antibiotic (ATB) administration has been shown to reduce febrile episodes and infections in neutropenic patients undergoing high dose chemotherapy or hematopoietic stem cell transplant (HSCT) in large meta-analyses (Gafter-Gvili, Cochrane, 2012; Kimura, J Infect Dis, 2014). This practice is supported by current guidelines in high-risk patients including those undergoing HSCT. However, ATB use has also been associated with adverse outcomes such as decreased microbiota diversity (Taur, Blood, 2014) and increased resistance (Magesic, Transpl Inf Dis, 2014). Recently, our group participated in a multicenter retrospective cohort of patients undergoing allogeneic (a) HSCT that demonstrated an increased incidence of acute graft versus host disease (aGVHD) and lower overall survival in patients receiving antibioprophylaxis (Routy, Oncoimmunology, 2017). To assess the impact of omitting prophylactic ATB, we used the sample from one of the participating centers (Hôpital Maisonneuve-Rosemont) to compare the incidence of bacteraemia and mortality in aHSCT patients who received prophylactic ATB during the pre-engraftment period to those who did not.
Methods
This retrospective study included patients undergoing aHSCT for hematological malignancy between January 2005 and December 2012 at our center. Exclusion criteria were prior aHSCT, syngenic and haploidentical HSCT. Antibioprophylaxis with fluoroquinolones or trimethoprim-sulfamethoxazole (TMP-SMX)was administered at initiation of the conditioning regimen in patients receiving a myeloablative (MA) and reduced intensity conditioning regimen and discontinued after engraftment. It was omitted in patients with fluoroquinolone or penicillin allergy and in patients receiving a non-myeloablative regimen. Prophylactic ATB were substituted for therapeutic ATB during episodes of febrile neutropenia and documented infection. Bacteraemia occurring during the pre-engraftment period (30 days or less after stem cell infusion) were recorded.
Results
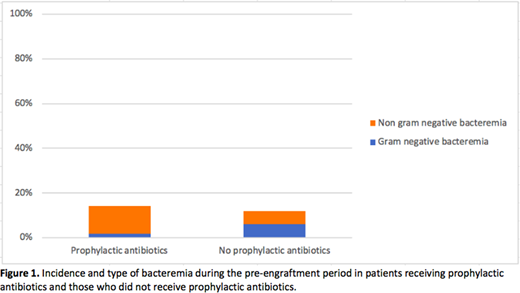
A total of 377 patients were included, of which 182 (48%) received prophylactic ATB and 195 (52%) did not. In the ATB group, 141 patients received ciprofloxacin, 17 moxifloxacin and 24 TMP-SMX. Baseline characteristics differed in both groups. The ATB group had a younger mean age with 45.2 ±12.3 years compared to 49.5 ±11.3 years (p<0.001) and a higher intensity conditioning regimen with 74% receiving a MA regimen compared with 36% (p<0.001). The incidence of bacteraemia was similar in both groups with 14.3% (95% CI 9.2-19.4%) in those receiving prophylactic ATB compared to 12.3% (95% CI 7.7-17.0%) in those who did not (OR 1.19, 95% CI 0.7-2.2, p 0.57). Expectedly, gram negative bacteremia was less likely (OR 0.24, 95% CI 0.07-0.84, p 0.026) in the ATB group, representing 11.5% of all bacteraemia compared to 52.2%. The incidence of bacteraemia was also reduced by prophylactic ATB in patients receiving a MA conditioning regimen (OR 0.48, 95% CI 0.25-0.96, p 0.036). There was no mortality associated to infectious causes during the pre-engraftment period in either group. Two patients died during the pre-engraftment period, one from hepatic failure and the other from acute respiratory distress syndrome in the context of veno-occlusive disease. Both were in the group that did not receive prophylactic ATB and received MA conditioning.
Discussion
In this study, the omission of prophylactic ATB did not increase the incidence of bacteraemia in patients undergoing aHSCT. However, the administration of prophylactic ATB did reduce bacteremia in those receiving MA conditioning regimens, as well as gram negative bacteraemia in all patients. Nevertheless, there was no impact on treatment related mortality at day 30 after stem cell infusion. Thus, the benefit of prophylactic ATB must be weighed against the higher risk of aGVHD and shorter overall survival observed in this sample in earlier studies (Routy, Oncoimmunology, 2017) and with the potential risk of selection and resistance. The limitations of this study include its retrospective design, the heterogenicity between the two groups and its omission of febrile episodes and infections without bacteraemia. Further data, preferably from a large prospective, randomised trial, would be useful to reassess the benefits and risks of prophylactic antibiotics in aHSCT.
Lachance:ExCellThera: Patents & Royalties: Royalities from sales of UM171.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal