Abstract
Background: In July of 2016, CMMI started a five year bundled payment program called OCM. Beneficiaries are attributed to practices providing their cancer care for a 6 month episode triggered by the administration or distribution of specified cancer drugs. The model provides risk adjustments to the target price based on risk factors such as age, chemotherapy and the receipt of certain treatments (radiation or bone marrow transplant). Target prices are adjusted by geographic region, novel therapy use, and a trend factor.
Multiple Myeloma was identified in our practice as a cancer type with high variance on expenditures after the first Performance Period within the model (July 2016-December 2016). Chemotherapy represented 52% of total episode expenditures with oral chemotherapy and hormone therapy representing 24%. The average cost of lenalidomide for one year is $115,000. The model adjusts for novel therapies, including new drugs approved by the FDA after December 31, 2014 with status lasting two years. However, literature demonstrates that this does not fully adjust for the high costs of novel therapies (Muldoon et at., Health Affairs, 2018). Unlike solid tumors, Multiple Myeloma staging may not improve risk adjustment and target price.
Methods: We analyzed the total cost of care of beneficiaries who triggered an OCM episode for Performance Period 1 (PP1). Beneficiaries were identified by diagnosis of Multiple Myeloma, and then segregated into cohorts of those who received lenalidomide and/or pomalidomide and those who did not. Observed vs. Expected (O/E) target price for each episode was determined for both cohorts comparing the actual episode expenditures and the target price per episode calculated by the Oncology Care Model. A two sample t-test was conducted followed by a linear regression to determine relation between drug days prescribed and O/E.
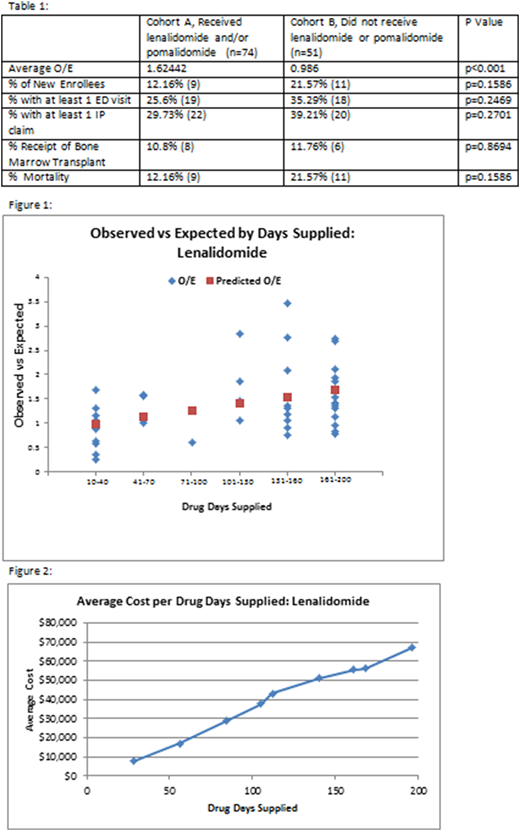
Results: There were 125 attributed beneficiaries with a Multiple Myeloma diagnosis who triggered an episode during PP1. The average O/E of the cohort which received the chemotherapy, Cohort A, was 1.624 compared to 0.986 for those that did not, Cohort B. The difference in average O/E in the two cohorts was 39% higher in Cohort A, p<0.001. There were no significant differences in the amount of inpatient claims, ED visits, or Bone Marrow Transplants between the two cohorts (Table 1). Figure 1 demonstrates the positive linear relationship (p<0.01, r=.40) between number of days supplied and O/E.
Discussion: This is the first report on the impact of lenalidomide and pomalidomide on the total cost of care in an OCM practice. The results demonstrate the lack of adequate adjustment to the CMS target price for episodes in which one or both of these drugs were prescribed. Lenalidomide and pomalidomide are first and second line drugs used both for induction and maintenance. Both drugs are frequently used for prolonged periods of time in patients and trigger more than one episode in OCM. Therefore, the use of these agents greatly affects the total cost of care against a target price that is not adequately adjusted. Academic Medical Centers that care for larger populations of multiple myeloma patients may be disproportionately affected and this will impede their success under the OCM methodology. Additional analysis similar to this will inform CMMI as to further refinements to the OCM adjusters.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal