Abstract
Background: Romiplostim (Nplate®) is a thrombopoietin (TPO) receptor agonist approved for the treatment of adult chronic immune thrombocytopenia (ITP). The formation of antibodies (Abs) against romiplostim may lead to a loss of response, and a theoretical risk exists that an immune response against romiplostim might lead to the formation of Abs that bind both romiplostim and native TPO. We therefore examined Abs against romiplostim and TPO in adult patients (pts) enrolled in clinical trials and in a global postmarketing registry based on spontaneously submitted requests for Ab testing.
Methods: The clinical trial population included adult pts (age ≥18 years at screening) with ITP who received ≥1 dose of romiplostim in any of 13 completed romiplostim clinical trials, including phase 1 (n=1), phase 2 (n=3), phase 1/2 (n=1), phase 3 (n=5), and open-label extension (n=3) studies. Duration of romiplostim treatment varied among the trials. The postmarketing global registry population included romiplostim-treated adult pts with ITP who had blood samples sent for Ab testing. Serum samples from the clinical trials and the registry were assessed for romiplostim and TPO binding Abs in a surface plasmon resonance (SPR)-based immunoassay, and samples positive for binding were assessed for neutralizing activity in a cell-based bioassay, as previously described (Jawa et al. Ann Hematol 2010). Pt characteristics were summarized for those who did and did not develop Abs in the clinical trials. Pts in the registry found to have romiplostim neutralizing Abs were to be followed every 3 months for up to 12 months. No formal statistical analyses were conducted.
Results: A total of 1046 romiplostim-treated pts from clinical trials were available for analysis. At baseline, 958 pts had romiplostim Ab results: 35 pts (3.7%) were positive for binding Abs and 1 pt (0.1%) was positive for neutralizing Abs. Post-baseline, 961 pts had romiplostim Ab results: 80 pts (8.3%) were positive for binding Abs and 4 pts (0.4%) were positive for neutralizing Abs, independent of the baseline result.
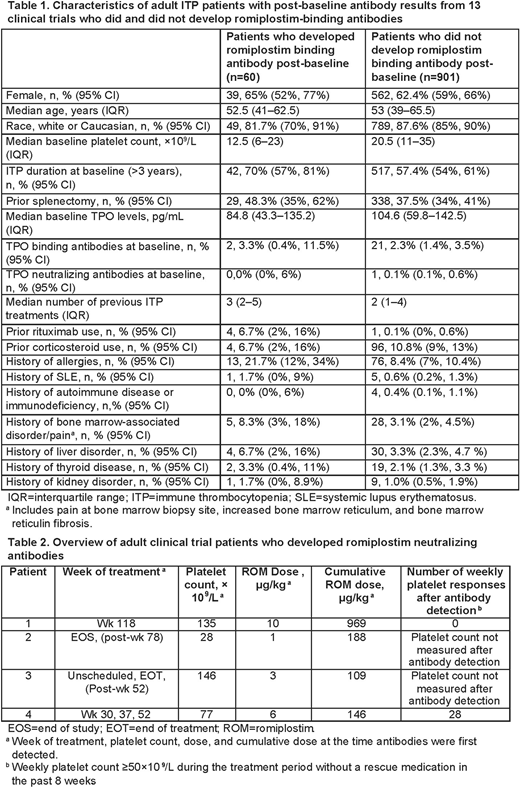
Sixty (6.2%) pts negative for anti-drug Abs at baseline developed romiplostim binding Abs during the clinical trials. Characteristics of the pts who did and did not develop romiplostim binding Abs are presented in Table 1. Development of romiplostim binding Abs was more frequent in pts with ITP duration >3 years at baseline (70.0% vs 57.4%), prior splenectomy (48.3% vs 37.5%), and a history of allergies (21.7% vs 8.4%). Pts who developed Abs also had lower baseline TPO levels (84.8 pg/mL vs 104.6 pg/mL), lower baseline platelet counts (12.5× 109/L vs 20.5 × 109/L), and a higher number of previous ITP treatments (median 3 vs 2).
Four of the 60 pts with romiplostim binding Abs developed romiplostim neutralizing Abs after treatment (0.38% of 1046 pts overall; Table 2). The emergence of neutralizing Abs did not appear to be related to romiplostim dose or platelet count. The neutralizing Abs were directed against the peptide component of romiplostim and did not bind native TPO.
Pts in the clinical trials were also tested for TPO Abs. At baseline, 956 pts had TPO Ab results: 31 pts (3.2%) were positive for TPO binding Abs and 1 pt (0.1%) was positive for neutralizing Abs. Post-baseline, 960 pts had TPO Ab results: 33 pts (3.4%) were positive for TPO binding Abs and no pts were positive for neutralizing Abs.
Of the 184 adult pts in the registry, 9 (4.9%) were positive for binding Abs: 5 were positive for romiplostim binding, 2 for TPO binding, and 2 for romiplostim and TPO binding. No predose Ab results were available for these pts. One pt received romiplostim for 11 months at a dose of 2 µg/kg and then experienced an abrupt fall in platelet count even as romiplostim dose was increased to 10 µg/kg. The pt tested positive for romiplostim binding and neutralizing Abs. Romiplostim was discontinued, and the pt was switched to alternative therapy.
Conclusions: An analysis of pts in 13 clinical trials shows that pts with indicators of more severe disease (longer duration of ITP, prior splenectomy, and a higher number of previous ITP treatments) may be at increased risk of developing romiplostim binding Abs. Development of romiplostim neutralizing Abs was uncommon in the clinical trials, and these Abs did not bind native TPO. Data from a postmarketing registry showed that the overall risk of clinically significant immunogenicity following exposure to romiplostim is low.
Barger:Amgen Inc.: Employment, Equity Ownership. Boshier:Sanofi: Other: rents a room to a Sanofi employee; Amgen Inc.: Employment, Equity Ownership. Kim:Amgen Inc.: Employment, Equity Ownership. Mytych:Amgen Inc.: Employment, Equity Ownership. Park:Amgen Inc.: Employment, Equity Ownership. Kuter:Amgen Inc.: Consultancy; Argenx: Consultancy; Rigel: Consultancy, Research Funding; Dova Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Principia: Research Funding; Protalex: Research Funding; Pfizer: Consultancy; Bioverativ: Consultancy, Research Funding; ONO: Consultancy; Syntimmune: Consultancy; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal