Abstract
Background: Prompt reversal of factor Xa (FXa) anticoagulation is essential in clinical situations with major bleeding or urgent surgery. Andexanet alfa (Andexxa, AnXa) is approved in the US for reversal of rivaroxaban or apixaban anticoagulation when necessary due to life-threatening or uncontrolled bleeding. In ANNEXA-4 interim report, ~85% of patients had good or excellent hemostasis after AnXa treatment (bolus + 2-h infusion). However, the minimum extent and duration of anticoagulation reversal required for a clinically relevant reduction in bleeding in humans have not been fully characterized.
Purpose: The impact of escalating doses of AnXa on reversing bleeding in a porcine polytrauma model with apixaban anticoagulation was investigated.
Method: After ethical approval, 40 anesthetized male pigs underwent a standardized polytrauma by blunt liver injury and bilateral femur fractures following 3-day apixaban administration (20 mg/day). 12 min post-trauma, animals were randomized (n=8 per group) to 5 groups: 250 mg bolus (AnXa 250 B), 500 mg bolus (AnXa 500 B), 250 bolus + 300 mg 2-h infusion (AnXa 250 B+), 500 mg bolus + 600 mg 2-h infusion (AnXa 500 B+), or vehicle (control). Blood loss (BL), hemodynamics and coagulation profiles were monitored over 5 h or until death; the primary outcome was total BL. Coagulation profiles consisted of apixabananti-FXa activity, global coagulation assays (PT, aPTT), rotational thromboelastometry (ROTEM) and thromboelastography (TEG). For differentiated risk assessment, thrombin generation, thrombin-antithrombin (TAT) and D-Dimer levels were measured. Data were analyzed by two-way ANOVA and log-rank tests (mean ± SD).
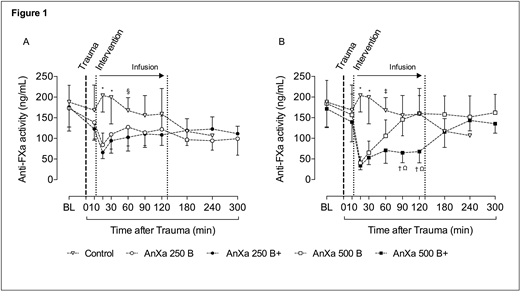
Results: Before the polytrauma, anti-FXa levels were 178.2 ± 52.7 ng/mL (similar among 5 groups). Trauma led to comparable BL at 12 min after injury (814 ± 79 mL). Compared with control (3464 ± 719 mL) and AnXa 250 B (3155 ± 364 mL; p=0.99 vs control), a significant reduction in total BL was observed in AnXa 250 B+ (2113 ± 297 mL; p<0.01 vs all other groups), AnXa 500 B (1427 ± 119 mL; p< 0.01 vs control, AnXa 250 B and AnXa 250 B+) and AnXa 500 B+ (1312 ± 213 mL; p=0.99 vs AnXa 500 B), with 100% survival. The mortality in the control group was 100%, whereas 62% of AnXa 250 B animals survived throughout the observation period. The reduction in total BL post-injury aligned with a dose-dependent reversal of anti-FXa activity (Figure 1). 5 min post-intervention, AnXa 500 B and AnXa 500 B+ regimens reduced the level of anti-FXa activity to 40.7 ± 14.6 and 33.8 ± 10.1 ng/mL, respectively. In contrast, reduction of anti-FXa activity to 84.3 ± 28.9 and 65.9 ± 14.9 ng/mL was seen in AnXa 250 B and AnXa 250 B+ regimens, respectively. This anti-FXa activity could be measured by the viscoelastic point of care tests on a specific FXa channel on a TEG. Activation of coagulation was indicated by dose-dependent increases in TAT and D-Dimer levels. This activation was in line with the recovery of the impaired thrombin generation following administration of AnXa. Clinically no adverse events were observed and the necropsy revealed no evidence of thromboembolic complications.
Conclusion: Dose-dependent reversal of apixaban by AnXa in this animal model was associated with decreased BL and mortality. The results indicate that AnXa regimens that decreased anti-FXa activity below a certain level (approx. 50 ng/mL), whether temporarily or sustained, was sufficient to prevent exsanguination in this animal model. This remaining low level of anticoagulation after apixaban reversal does not prevent activation of coagulation, allowing for sufficient hemostasis. Our results warrant further investigation of the extent and duration of reversal of FXa inhibition required to achieve adequate hemostasis in humans.
Figure 1: Anti-FXa activity in control, AnXa 250 B and AnXa 250 B+ groups (A). Anti-FXa activity in control, AnXa 500 B and AnXa 500 B+ groups (B). Initially n=8 per group. *P<0.05 vs. all other groups; Ω<0.05 vs. control; § P<0.05 vs. AnXa 250 B+; †P<0.05 vs. AnXa 500 B; ‡P<0.05 vs. AnXa 500 B+. BL: baseline (after 3-days oral apixaban administration and before the polytrauma)
Grottke:Pfizer: Honoraria; Sanofi: Honoraria; Octapharma: Consultancy, Research Funding; Portola: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding. Conley:Portola: Employment. Rossaint:CSL Behring: Honoraria; Boehringer Ingelheim: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal