Abstract
BACKGROUND
Although the overall survival for children with AML has serially improved due to better supportive care, intelligent use of transplant in 1st complete remission (CR1), and better salvage in CR2, the relapse rate (RR) in CR1 remains unacceptably high at 30-35%. Increasing the intensity of induction chemotherapy may improve outcome. Intensifying anthracycline dosage in induction has been reported to improve outcome in adult AML1, but this remains unproven in children in whom anthracycline-related cardiotoxicity is a major concern. Gemtuzumab ozogamicin (GO), the anti-CD33-calicheamicin conjugated antibody is the most promising new drug being explored in AML treatment presently. Cardiac myocytes do not express CD33 and therefore GO may enable intensification of induction chemotherapy without increasing cardiotoxicity. In children, GO is given as a single 3 mg/m2 dose, however the optimum dose, schedule and timing when combined with chemotherapy remains uncertain.
METHODS
A dose finding study (DFS) to establish the optimum tolerated number of 3 mg/m2 GO doses that can be combined with induction treatment: cytarabine plus mitoxantrone or liposomal daunorubicin (LD) was embedded within MyeChild 01; an international randomised phase III study for children with AML and high risk MDS.
This major DFS recruited pts aged ≥12 months and <18 years. A minor DFS recruiting pts aged ≥12 weeks and <12 months is ongoing. The dose schedule for GO 3 mg/m2 was studied in course 1 only: cohort 1: GO d4, cohort 2; GO d4 and 7 and cohort 3; GO d4, 7 and 10.
High-risk patients (defined by poor risk cytogenetics and/or a failure to achieve remission) received fludarabine, cytarabine and idarubicin for course 2. All other patients received a second course of the randomised therapy.
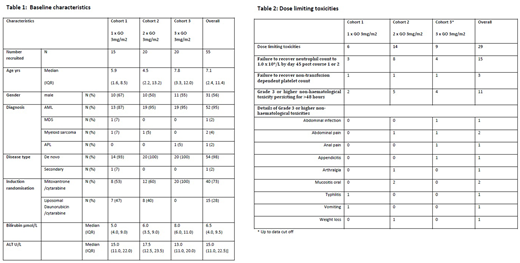
Progression to next cohort was determined by an independent data monitoring committee (DMC) taking into account known toxicities of the induction chemotherapy. Dose limiting toxicities (DLTs) were defined as neutrophils ≤1.0 x109/l by d45 post course 1 or 2, non-transfusion dependent platelets ≤80 x109/l by d45 post course 1 or 2 due to documented bone marrow aplasia/hypoplasia and non-haematological events: death from any cause other than AML, veno-occlusive disease (VOD) and any grade ≥3 non-haematological toxicity persisting for >48 hours without resolution to grade ≤2 with specific protocol defined exceptions.
RESULTS
At data cut-off (27 July 2018), 55 pts were recruited, of which 51 were AML, 1 MDS, 2 myeloid sarcoma and 1 APL (not dosed as ineligible) (Table 1). The chemotherapy randomisation was not balanced in cohort 3 due to suspension of the randomisation when LD became unavailable due to a manufacturing failure; 39 pts received cytarabine with mitoxantrone and 15 cytarabine with LD. Post-course 1, 22 (40%) were stratified as high risk.
54 patients were dosed with GO. In cohort 1; 15 pts received GO 3 mg/m2 on d4; 5 were added as an expansion cohort on the recommendation of the DMC due to 2 patients having unevaluable haematological toxicities. Cohort 2; 20 pts received GO 3 mg/m2 on d 4 and 7. Both cohorts have completed the DLT evaluation period. In cohort 3; 19 pts received GO 3 mg/m2 on days 4, 7 and 10. The DLT evaluation period is near completion for all cohort 3 pts and the data will be presented.
Table 2 presents grade ≥3 AEs at data cut off. 29 DLTs were reported, 6 in cohort 1 and 14 in cohort 2. In cohort 3, 9 DLTs are reported but 13 pts are still in the DLT evaluation period. The commonest DLTs were failure to recover either neutrophil or platelet count by d45 post course 1 or 2. DMC review confirmed the reported DLTs for cohorts 1 and 2 were consistent with expected toxicity from the combined chemotherapy alone. Six cases of VOD were reported, all outside the DLT evaluation period, none were fatal.
CONCLUSIONS
The preliminary findings indicate that 2 doses of 3 mg/m2 GO combined with induction chemotherapy is well tolerated in this patient population, consistent with data from adult AML studies. The DMC concluded for cohorts 1 and 2 that the reported toxicities, including myelosuppression and non-haematological toxicities did not exceed the expected events associated with intensive induction chemotherapy in the absence of GO and there was a notable absence of significant DLTs. There was no apparent difference in AEs between mitoxantrone or LD arms. Cohort 3 DLT reporting is ongoing and the data will be presented.
Lowenberg, NEJM 2009; Ohtake, Blood 2011.
Gibson:Pfizer: Research Funding; Galen: Research Funding. Houlton:Pfizer: Research Funding. Baruchel:Novartis: Membership on an entity's Board of Directors or advisory committees; Shire: Research Funding; Celgene: Consultancy; Amgen: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Travel, accommodations or expenses; Servier: Consultancy; Roche: Consultancy. Wheatley:Pfizer: Research Funding. Kearns:Galen: Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal