Abstract
Background: Reactivation of hepatitis B virus (HBV) is a well-recognized complication in patients with chronic HBV infection undergoing cytotoxic or immunosuppressive therapy. Currently, BCR-ABL1 tyrosine kinase inhibitors (TKIs) have been a standard of first-line treatment for chronic myeloid leukemia (CML) about last two decades. As there have been several case reports of HBV reactivation in CML patients treated with TKIs, the test for hepatitis B infection before initiating TKIs is recommended. Therefore, this study aimed to evaluate the risk of HBV reactivation in a large cohort of CML patients on TKIs treatment.
Methods: We retrospectively reviewed the medical records of 1817 patients, who were diagnosed with CML and had available HBV serology between 2001 and 2017 from St. Mary's Hospital, Seoul, Korea.
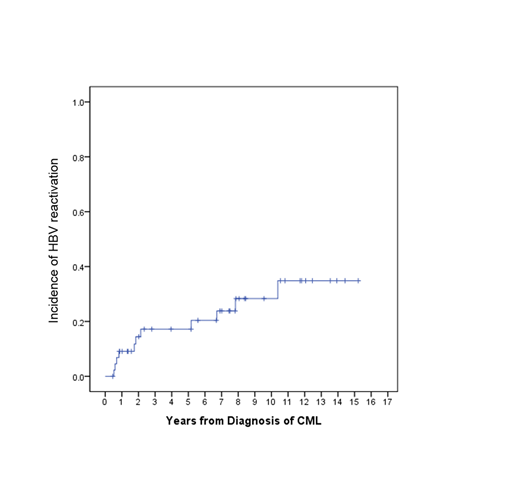
Results: Among 1817 patients, 76 patients (4.2%) were HBs-antigen (HBs-Ag) positive and 1741 (95.2%) were HBs-Ag negative. 302 patients (17.3%) of 1741 HBs-Ag negative patients were HBc-antibody positive indicating past infection of HBV. After exclusion of 7 patients who did not receive TKIs treatment, 69 patients were analyzed for HBV reactivation. The median age at diagnosis of CML was 42 (range 21-73) years and 44 (64%) were males. Median duration of follow-up from the recognition of HBs-Ag was 66.7 months (range, 2.4 - 192.7 months). Of 69 patients, a patient had been on antiviral therapy for chronic HBV infection before the diagnosis of CML. Eighteen patients (26% of 68) received antiviral prophylaxis prior to or concomitantly with the initiation of TKIs. Additional 4 patients were simultaneously initiated antiviral treatment with TKIs due to the evidence of active HBV infection such as the high titer of HBV DNA or elevated level of Alanine aminotransferase. Among 46 patients who did not receive antiviral prophylaxis, 12 patients (26%; 95% CI, 12-36%) eventually required antiviral treatment due to the development of active HBV hepatitis while on TKI therapy. HBV reactivation according to each TKI treatment was developed in 7 patients with imatinib, 2 patients in dasatinib, 1 patient in nilotinib, and 1 patient in radotinib therapy. One patient developed HBV reactivation permanently discontinued TKI therapy. Median time to HBV reactivation from CML diagnosis was 22.1 (range, 6.3-124.6 months) months. Median duration of antiviral therapy of 35 patients who received antiviral therapy was 38.2 months (95% CI, 25.7 ~ 50.7 months). 9 patients were off antiviral therapy at the last follow-up, 5 patients from prophylaxis group and 4 patients from treatment group. There was no difference in the duration of antiviral treatment between prophylactic and treatment groups. The initial choice of antiviral therapy as prophylaxis or treatment were entecavir for 14 (41% of 34), lamivudine for 8 (24%), tenofovir for 8 (24%), telbivudine for 3 (9%), and adefovir for 1 (3%).
Conclusion: We evaluated HBV reactivation in a large cohort of HBs-Ag positive patients with CML from a single center, who received TKIs treatment. The reactivation rate of HBV was high at 26% without antiviral prophylaxis, which strongly support the routine screening of HBV serology prior to initiation of TKI therapy to identify HBs-Ag positive patients and the close monitoring of hepatic functions and HBV serology during TKI therapy. Given the high incidence of HBV reactivation with TKI treatment, concomitant antiviral prophylaxis with TKIs should be considered in HBs-Ag positive patients receiving TKI treatment.
Kim:Ilyang: Research Funding; Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal