Abstract
Introduction: TP53 mutant (mTP53) MDS and AML, accounting for 5-10% of de novo MDS and 25-30% of therapy related MDS (t-MDS), represent a distinct molecular cohort with inferior outcomes. Hypomethylating agents (HMA) are preferred treatments for patients (pts) with these mutations, although with CR rates of only 20-30% and median OS of 6-12 months. APR-246 is a novel, first-in-class small molecule that selectively induces apoptosis in mTP53 cancer cells through mutant p53 protein re-activation by restoring the wild-type conformation, with single agent activity in mTP53 AML. We report the planned, completed Phase 1b results of APR-246+ azacitidine (AZA) in mTP53 MDS/AML.
Methods: This is a multicenter Phase 1b/2 trial of APR-246+AZA in HMA naïve mTP53 MDS and oligoblastic AML (≤ 30% blasts) pts ≥ 18 years of age. Pts received APR-246 in a 3+3 dose escalation design (50, 75, 100 mg/kg lean body weight (equivalent to 4500mg fixed dose based on PK studies)) IV daily over 4 days in a lead-in phase (days -14 to -10) followed by the same dose of APR-246 (days 1-4) + AZA 75 mg/m2 SC/IV over 7 days (days 4-10 or 4-5 and 8-12) in 28 day cycles. The primary objective was to define safety and the recommended Phase 2 dose (RP2D), with AEs graded by CTCAE v4.03 and DLT assessment over 6 weeks. Secondary objectives included response by IWG 2006 criteria as well as serial next generation sequencing (NGS) and p53 IHC for evaluation of clonal suppression and remission depth as predictors of outcomes. For minimal residual disease (MRD) analysis, a custom target-capture NGS assay was developed using unique molecular Identifiers for error correction with a limit of detection of 0.1% with results validated by pt specific digital droplet PCR (ddPCR). Nanostring nCounter RNA expression analysis was conducted on a panel of 770 genes after the lead-in phase to assess transcriptional effects induced by APR-246.
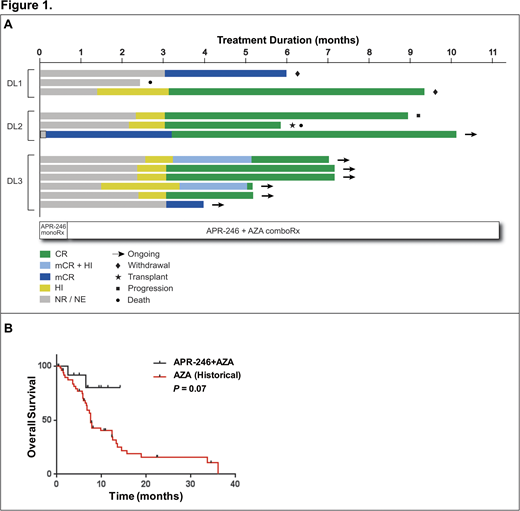
Results: As of July 30, 2018, 12 pts (42% male; median age 66 years (39-73)) were enrolled. Three pts had AML-MRC and 9 had MDS; all pts had poor risk cytogenetics (17% poor, 83% very poor) and higher risk disease by IPSS-R (25% high, 75% very high). T-MDS occurred in 5 pts (42%) and 7 pts (58%) were transfusion-dependent at baseline. Median BM blasts were 9% (4-30). Eleven of 12 pts (92%) had a TP53 missense mutation in the DNA binding domain with multiple mutations in 4/12 pts (33%). For 9/12 pts (75%), TP53 was the sole mutation. Median time on study is 176 days (41-298) with 7 pts ongoing. Treatment (Tx) related AEs during the lead-in phase (all G1) included nausea (n=5), neuropathy (n=5), decreased appetite (n=2), and dizziness (n=2) which were all transient. Tx related AEs occurring in > 1 pt in the combination phase included nausea/vomiting (n=6), dizziness (n=3), headache (n=3), neuropathy (n=3), fall (n=2), pruritus (n=2), thrombocytopenia (n=6), neutropenia (n=5), and leukopenia (n=4); all G1/G2 except cytopenias (G3/G4). No DLTs have occurred to date.
Eleven of twelve pts were response evaluable with 1 pt discontinuing tx prior to 1st disease assessment (Fig 1A). ORR by IWG was 100% (11/11) with 9 CR (82%) and 2 marrow CR (mCR; 18%). Median time to first response was 70 days (4-91) and one CR patient achieved mCR and partial cytogenetic response after APR-246 lead-in prior to combination therapy. All CR pts had high p53 positivity by IHC at baseline (25-80%) which normalized on serial assessment with the 2mCR pts having <5% p53+ at baseline. Serial NGS with a variant allele frequency (VAF) cutoff of 5% was negative in 73% of patients (8/11). In NGS negative pts, MRD analysis, validated by ddPCR, was performed with a median VAF of 0.3% (0.1%-3.1%) at best molecular response. Enriched pathway analysis via Reactome following APR-246 lead-in phase showed transcriptional activation of p53 targets (FDR = 9.16E-09), including pathways involved in cell cycle arrest, apoptosis, DNA repair, and regulation of TP53 activity. At a median follow up of 7 months, the median OS or PFS has not been reached. In comparison to a internal historical cohort of 51 mTP53 MDS/AML treated with AZA alone, APR-246+AZA had a trend for improved OS (NR vs 7.6months; HR 0.30, P=0.07; Fig 1B).
Conclusions: APR-246+AZA combination is well tolerated in mTP53 MDS/AML. Responses have been achieved in all evaluable pts (82% CR) accompanied by deep molecular and durable remissions. The RP2D of APR-246 is a fixed dose of 4500mg days 1-4 in combination with AZA and phase 2 accrual has begun.
Sallman:Celgene: Research Funding, Speakers Bureau. Sweet:Agios: Consultancy; Phizer: Consultancy; Agios: Consultancy; BMS: Honoraria; Celgene: Honoraria, Speakers Bureau; Jazz: Speakers Bureau; Celgene: Honoraria, Speakers Bureau; BMS: Honoraria; Phizer: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Astellas: Consultancy; Jazz: Speakers Bureau; Astellas: Consultancy. Cluzeau:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Speakers Bureau; Sanofi: Speakers Bureau; Menarini: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sekeres:Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Roboz:Orsenix: Consultancy; Cellectis: Research Funding; Eisai: Consultancy; Astex Pharmaceuticals: Consultancy; Argenx: Consultancy; Pfizer: Consultancy; Bayer: Consultancy; Eisai: Consultancy; Sandoz: Consultancy; Jazz Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Otsuka: Consultancy; Bayer: Consultancy; Aphivena Therapeutics: Consultancy; Celltrion: Consultancy; Argenx: Consultancy; Celgene Corporation: Consultancy; Daiichi Sankyo: Consultancy; Celltrion: Consultancy; Sandoz: Consultancy; Astex Pharmaceuticals: Consultancy; Aphivena Therapeutics: Consultancy; Orsenix: Consultancy; Otsuka: Consultancy; Roche/Genentech: Consultancy; Roche/Genentech: Consultancy; Janssen Pharmaceuticals: Consultancy; Daiichi Sankyo: Consultancy; Novartis: Consultancy; AbbVie: Consultancy; Janssen Pharmaceuticals: Consultancy; Celgene Corporation: Consultancy; AbbVie: Consultancy; Cellectis: Research Funding. Bhagat:Genoptix: Employment. Tell:Aprea Therapeutics: Employment. Fenaux:Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Roche: Honoraria. List:Celgene: Research Funding. Komrokji:Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal