Abstract
Based on the clinical success observed in acute lymphoblastic leukemia (ALL) with chimeric antigen receptor engineered T (CAR T), we hypothesized that combining the specificity of a CAR with the innate allo-reactivity of KIR-mismatched NK cells might provide a powerful tool for adoptive cell therapy. The use of a third-party bank of CAR-NK cells offers the advantage of an immediate availability to be exploited in the allogenic setting and could be associated with a lower toxicity profile than CAR-T cells.
In order to overcome regulatory and manufacturing hurdles associated with generation of CAR-NK cells, we developed a feeder-free culture resulting in a 3.2-log expansion after 20 days of culture. Specifically, natural cytotoxicity receptors (NCR) expressed on NK cells are stimulated in the presence of pleiotropic cytokines and expanded in GMP grade bioreactors. Expanded NK cells from healthy donors preserve a high percentage of CD56+ CD57- cells (85±13%), associated with high proliferative capability, and maintain the surface expression and the responsiveness of NCR and CD16.
We proved that NK cells generated from 10 different healthy donors have high ability to recognize and eliminate different tumor types, including acute myeloid leukemia (AML) and ALL.
After genetic modification with a retroviral vector encoding a CAR specific for CD19 antigen, transduction of activated NK cells averaged 38%±15% and the CAR.CD19 expression was stable over extended in vitro culture (60 days). Detailed phenotypic characterization of CAR-NK cells showed that CAR expression was not limited to the more mature NKG2A-/KIR+ cells, but rather was distributed across different NK subsets.
We also demonstrated that NK and CAR-NK cells display significant anti-leukemia activity towards CD19+ leukemia and lymphoma cell lines (LCL 721.221, DAUDI and BV173) and primary blasts obtained from patients with B-cell precursor ALL (Bcp-ALL). Co-culture experiments using a 1:5 E/T ratio, showed that, while the anti-tumor activity was already remarkable with non-modified effector NK cells (60±30%, 71±33% and 54±23% of residual LCL 721.221, DAUDI and BV173 cells, respectively; p<0.05 vs T cells), it reached the highest level when CAR-NK cells were used as effectors (7±9%, 16±30% and 22±16% of residual LCL 721.221, DAUDI and BV173 cells, respectively; p<0.05 vs non-transduced NK cells). Importantly, INF-g production was significantly lower upon CAR-NK activation compared to CAR-T cells (DAUDI 384±194 ng/ml vs 1860±678 ng/ml, p=0.002). Functional analysis on primary Bcp-ALL blasts, demonstrate that CAR-NK cells exert high degree of leukemia control (on average 2.1±2% vs 5.4±1.6% with non-modified NK cells as effectors; p=0.04).
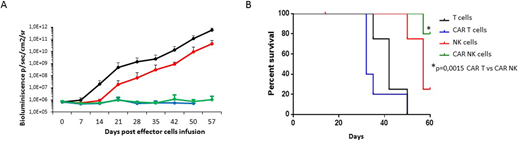
An in vivo model of leukemia xenograft immunodeficient mice was used to evaluate whether CAR-NK cells are associated with a lower toxicity profile compared to CAR-T cells. While the in vivo antileukemia activity was superimposable between CAR-T and CAR-NK cells (mouse bioluminenscence at 20 days, 4.9x105 vs 6.6x105 photons/second, respectively; p=n.s. Figure A), mice treated with two i.v. infusions (day 0 and day 15) of 10x106 CAR.CD19 NK cells had a 100% overall survival (OS of 5 out of 5 mice) at 50 days compared to 20% of mice (1 out of 5) receiving 10x106 CAR.CD19 T cells (Figure B; p=0.01). Cytokine plasma level monitoring, performed on day +7 and +30 after effector cell infusion in the absence of leukemia persistence (as evidenced by a lack of bioluminescence signal), showed that mice engrafted with CD19+ leukemia and treated with CAR.CD19-NK cells have lower levels of circulating hIFN-g cytokine compared to mice treated with CAR.CD19-T cells at both day 7 (42±82 vs 330±346 ng/ml; p=0.05) and day 30 (0.9±0.7 vs 4148±667 ng/ml; p=0.05).
These in vitro and in vivo data demonstrate the feasibility of clinical scale feeder-free expansion of non-modified NK cells and stably transduced CAR-NK cells. Both non-modified and gene-modified cells were capable of significant tumor killing, suggesting a multi-modal adoptive cell approach to treatment of leukemia. Since NK cells have been shown to be safely used in third-party setting (St. Jude Children's Research Hospital, USA; NCT00640796), we suggest that ex-vivo expanded, feeder-free NK cells can be universally applied for 'off-the-shelf' immuno-gene-therapy, and that their innate allo-reactivity can be safely harnessed to potentiate allogeneic cell therapy.
Locatelli:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi: Honoraria; Bellicum: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal