Abstract
Background: Marginal zone Lymphomas (MZL) are indolent B cell non hodgkin Lymphoma (NHL) and include splenic, nodal and extranodal subtypes (SMZL, NMZL, ENMZL). When therapy is needed in symptomatic patients, standard treatment usually requires systemic immunochemotherapy (ICT). Although the outcome of MZL is generally measured in decades a high heterogeneity of clinical behaviour exists that warrants the identification of accurate prognostic features to better estimate the risk of relapse, progression or death in the individual patient. Recently the analysis of progression free survival (PFS) was used to identify clinically useful endpoints in B-cell NHLs, with PFS at 24 (PFS24) months identified to stratify overall survival (OS) in follicular NHL. Here we examined the ability of PFS24 to predict subsequent OS in a large, multinational MZL cohort as part of the NF10 observational multicentric international study promoted by Fondazione Italiana Linfomi (FIL).
Methods: The NF10 Project was started in 2010 as a prospective registry specifically conceived to investigate the prognosis of Indolent Non-Follicular B-Cell Lymphomas (INFL). The registration of clinical, laboratory data, treatment and outcome details of consecutive adult patients with newly diagnosed INFL was available at a dedicated website. All patients with a histologic confirmed diagnosis of INFL also including MZL were eligible with no exclusion criteria. Patients were followed based on local institution guidelines, and PFS was defined as time from the date of pathologic diagnosis to progression, re-treatment, or death due to any cause. PFS24 was calculated only for patients requiring immediate therapy and was defined as being alive and progression-free 24 months from diagnosis. Subsequent OS was defined as time from achieving PFS24 or time from progression in patients failing to achieve PFS24 (progression within 24 months of diagnosis).
Results: Between July 2010 and July 2018, 1.253 INFL cases have been registered by 65 centres in Europe and South America. MZLs were 677 (54%): 283 ENMZL (43%), 221 SMZL (32%), 69 NMZL (10%); 104 cases were classified as disseminated MZL (Diss-MZL 15%) due to the lack of a clear pattern of organ involvement. Median age was 66 years (range 27-93); Ann Arbor stage was III-IV in 79%; 14% had B symptoms, 6% had ECOG performance status (PS) >1, lactate dehydrogenase (LDH) and β2-microglobulin were above upper normal limit (UNL) in 26% and 56% of cases respectively. Bone marrow involvement was present in 67%, positive HCV and HBV serology was found in 8% and 18% of cases respectively.
For the current study we identified 400 patients with MZL for whom immediate therapy was planned right after lymphoma diagnosis. Patients with immediate therapy were 59% of all MZL. Rituximab (R) combined with chemotherapy was used in 332 (82%): R plus bendamustine (RB) in 142 (36%), R plus alkylating agents (R-alk) in 101 cases (25%) (mostly ENMZL), R plus CHOP in 50 (12%) (mainly NMZL and DissMZL); R monotherapy was used in 36 (9%).
The median follow-up was 38 months (range 1-83). For treated pts 3y-PFS was 79% and 3y-OS was 90%; progressive disease was the cause of death in 47% of all cases.
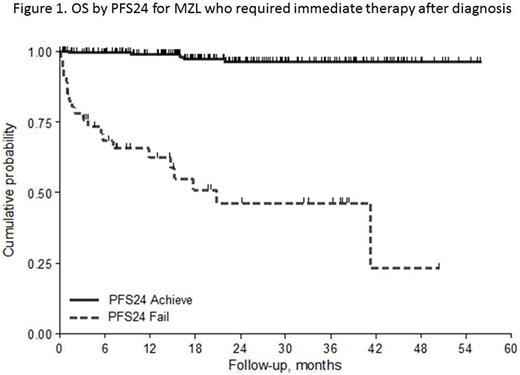
The percentage of patients who failed to achieve PFS24 was 20% with a lower frequency in the subtypes NMZL and ENMZL (13%) compared to the group of SMZL and DissMZL (24%, p=0.015). Three-year OS for patients with progression within the first 24 months was 46% (95%CI 28-63%) with a HR of 28.3 (95%CI 10.6 - 75.6) when compared with patients without early relapse (96%, 95%CI 91-98%). When PFS24 was adjusted by IPI in all cases and by HPLL (Montalbán et al, BJH 2012) in SMZL, the PFS24 retained its prognostic role (p<0.001).
The prognostic role of PFS24 was confirmed in ENMZL, SMZL and Diss-MZL subgroups (Figure 1). At present, in NMZL there were too few events to do an inference. At univariate logistic analysis predictive factors for PFS24 were the subtypes group SMZL/Diss MZL, poor ECOG PS, hemoglobin <9.5g/dl, BM involvement, platelets counts < 80.000/mmc, low albumin.
Conclusions: Assessment of PFS24 predicts subsequent outcome in MZL. The prognostic role of PFS is confirmed in both ENMZL and SMZL. Similarly to FL also in MZL, PFS24 should be considered as a surrogate for OS in clinical research and for patients management.
Luminari:Servier: Consultancy; Gilead: Consultancy; Celgene: Consultancy; Roche: Consultancy; Sandoz: Consultancy. Marcheselli:Fondazione Italiana Linfomi (FIL) Onlus: Employment. Annibali:Celgene; Takeda; Amgen, Janssen Cilag: Honoraria. Gaidano:Morphosys: Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal