Abstract
Background: MCL is a subtype of NHL that remains largely incurable with conventional therapies; BR is standard first-line therapy. Acalabrutinib is a potent, highly selective, covalent Bruton tyrosine kinase inhibitor with minimal off-target activity. Acalabrutinib was approved by the FDA in October 2017 for patients with R/R MCL and ≥1 prior therapy. This ongoing, multicenter, open-label Phase 1b study assessed the safety and efficacy of acalabrutinib + BR in patients with TN or R/R MCL.
Methods: Patients aged ≥18 y with ECOG PS ≤2 and adequate organ function were eligible; R/R patients had received ≥1 prior therapy. Patients received oral acalabrutinib 100 mg twice daily, plus B 90 mg/m2 intravenously (IV) on days 1 and 2 and R 375 mg/m2 IV on day 1 in each 28-day cycle. Acalabrutinib was given until disease progression (PD) or intolerance; BR was repeated every 28 days for up to 6 cycles. Patients with TN MCL who achieved partial or complete response (CR) received rituximab maintenance therapy (375 mg/m2 every other cycle for up to 12 doses starting on cycle 8). Dose-limiting toxicity (DLT) was evaluated in the first 6 patients per cohort (TN or R/R) after completing 1 cycle. If <2 patients had DLTs, cohorts were expanded. The primary endpoint was safety of acalabrutinib + BR. Secondary endpoints were overall response rate (ORR), duration of response (DOR) and progression-free survival (PFS). Pharmacokinetic evaluation was exploratory.
Results: A total of 38 patients enrolled (TN, n=18; R/R, n=20); 63% were men; 55% were ≥65 y. At baseline, 100% of TN and 95% of R/R patients had ECOG PS ≤1; 89% and 95% had Ann Arbor stage IV disease, 44% and 60% had intermediate-risk and 11% and 15% had high-risk simplified MCL International Prognostic Index scores, respectively. The median number of prior therapies in the R/R cohort was 2 (range 1-4) and 45% were refractory (stable disease or PD) to most recent prior treatment. As of 4 May 2018, median time on study was 17.6 mo (range 0.6-23.1) for TN and 14.2 mo (range 1.2-23.6) for R/R patients; 78% of TN and 50% of R/R patients completed 6 cycles of BR with acalabrutinib.
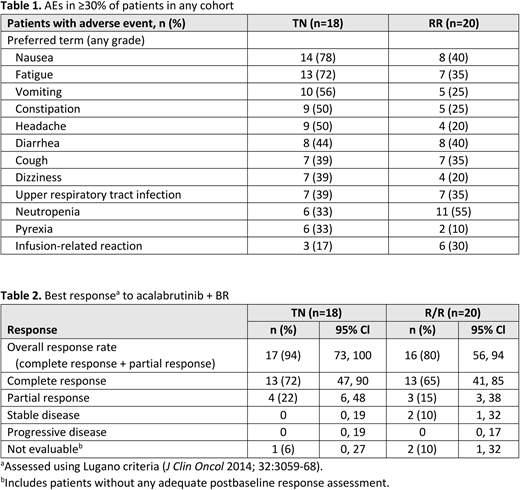
Across both cohorts, no DLTs were observed. Adverse events (AEs) of any grade in ≥30% of patients in any cohort are in Table 1.
In the TN cohort, Grade ≥3 AEs in ≥10% of patients were neutropenia (33%) and pneumonia (11%). Serious AEs (SAEs) occuring in ≥2 patients included pneumonia (Grade 3, n=2 [11%]) and pyrexia (Grade 1, n=2 [11%]). One patient had pulmonary alveolar hemorrhage (Grade 4, investigator assessed as related to acalabrutinib) leading to discontinuation of study treatment (acalabrutinib + BR). There were 3 deaths: 1 patient had Grade 5 pneumonitis (investigator assessed as related to acalabrutinib; 24 days after last dose), 1 died of unascertained cause, and 1 died of unknown cause.
In the R/R cohort, Grade ≥3 AEs (in ≥10%) were neutropenia (50%); diarrhea, leukopenia, decreased neutrophil count, pneumonia and thrombocytopenia (10% each). Pneumonia was the only SAE to occur in ≥2 patients (n=3; 1 Grade 1, 2 Grade 3). Three patients had major hemorrhage; all were Grade 3 AEs and investigator assessed as unrelated to study treatment (subdural hematoma [after a traumatic fall], large intestinal ulcer hemorrhage [on study day 2], gastrointestinal hemorrhage [duodenal tumor found on endoscopy]). One patient had Grade 3 angina pectoris (unrelated) leading to discontinuation of study treatment (acalabrutinib + BR). There were 4 deaths: 1 patient had Grade 5 AE cerebrospinal meningitis (investigator assessed as unrelated to acalabrutinib; 123 days after last dose), 1 died of unascertained cause, and 2 had PD.
No patients had cytomegalovirus infection, pneumocystis jiroveci pneumonia, or atrial fibrillation during the study.
In the TN cohort, the ORR was 94%, with a CR rate of 72% (Table 2); median DOR and median PFS were not reached (NR). In the R/R cohort, ORR was 80% and CR rate was 65%; median DOR was 15.0 mo (95% CI: 12.2, NR) and median PFS was 16.6 mo (95% CI: 14.2, NR).
There were no clinically meaningful differences in steady-state acalabrutinib exposure when administered with or without BR (n=12 [TN, n=6; R/R, n=6]).
Conclusion: Combination therapy with acalabrutinib + BR showed an acceptable safety profile, with no meaningful changes in acalabrutinib exposure. The high CR rate with acalabrutinib + BR in both TN and R/R MCL further supports a phase 3 randomized study of acalabrutinib + BR versus BR in TN MCL (NCT02972840).
Phillips:Bayer: Consultancy; Seattle Genetics: Consultancy; Pharmacyclics: Consultancy, Research Funding; Abbvie: Research Funding; Genentech: Consultancy; Gilead: Consultancy. Smith:Portola: Research Funding; Merck Sharpe Dohme and Corp: Consultancy, Research Funding; Acerta Pharma BV: Research Funding; Pharmacyclics: Research Funding; Genentech: Research Funding; Seattle Genetics: Research Funding. Jurczak:Afimed: Research Funding; BeiGene: Research Funding; Celgene: Research Funding; Epizyme: Research Funding; Gilead: Research Funding; Janssen: Research Funding; Nordic Nanovector: Research Funding; Merck: Research Funding; Morphosys: Research Funding; Pharmacyclics: Research Funding; Servier: Research Funding; Roche: Research Funding; TG Therapeutics: Research Funding; Gilead: Consultancy; Janssen: Consultancy; Sandoz-Nowartis: Consultancy; AstraZeneca/Acerta: Consultancy, Research Funding; European Medicines Agency: Consultancy. Robak:AbbVie, Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Stevens:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Farber:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Research Funding; Acerta: Research Funding; Pharmacyclics: Research Funding; Genentech: Honoraria, Research Funding, Speakers Bureau; Charles M. Farber, MD, PhD, LLC-Medical legal consulting: Consultancy; ummit Medical Group-MD Anderson Cancer Center: Employment; Gilead: Honoraria; Seattle Genetics: Honoraria, Speakers Bureau. Pagel:Pharmacyclics, an AbbVie Company: Consultancy; Gilead: Consultancy. Maddocks:Merck: Research Funding; Pharmacyclics: Research Funding; Novartis: Research Funding; Pharmacyclics/Janssen: Honoraria; AstraZeneca: Honoraria; Teva: Honoraria; BMS: Research Funding. Flinn:Janssen: Research Funding; Trillium: Research Funding; Calithera: Research Funding; Celgene: Research Funding; Forma: Research Funding; Verastem: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; BeiGene: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding; Novartis: Research Funding; Forty Seven: Research Funding; Constellation: Research Funding; Genentech: Research Funding; Agios: Research Funding; Infinity: Research Funding; Merck: Research Funding; Verastem: Consultancy, Research Funding; TG Therapeutics: Research Funding; Portola: Research Funding; Gilead: Research Funding; Kite: Research Funding; Incyte: Research Funding; ArQule: Research Funding; Curis: Research Funding. Jedrzejczak:BMS: Research Funding; Astellas: Research Funding; Onconova: Research Funding; Amgen: Research Funding; Polish Ministry of Higher Education and Science: Research Funding; Takeda: Honoraria, Research Funding; Abbvie: Honoraria; Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria; Novartis: Honoraria, Research Funding; Janssen Cilag: Honoraria; Polish Parliament: Consultancy; Polish Ministry of Health: Consultancy; Medical University Warsaw, Central Hospital Warsaw: Employment; Astex: Research Funding; AstraZeneca: Research Funding. Goy:Seattle Genetics: Research Funding; Genentech: Research Funding; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Acerta: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics/J&J: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Hackensack University Medical Center: Employment; COTA: Membership on an entity's Board of Directors or advisory committees. Zinzani:MSD: Honoraria, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Zaucha:Celgene: Honoraria; Amgen: Honoraria; Roche: Honoraria; Takeda: Honoraria. Coleman:Gilead: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Speakers Bureau; Kite Pharmaceuticals: Equity Ownership; Celgene: Consultancy, Research Funding, Speakers Bureau; Merck: Research Funding; Bayer: Consultancy, Research Funding, Speakers Bureau; Incyte: Research Funding. Chen:Acerta Pharma: Employment; AstraZeneca: Equity Ownership; Merck: Equity Ownership. Lee:Acerta Pharma: Employment. Liang:Acerta Pharma: Employment. Seto:Acerta Pharma: Employment. Wang:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Research Funding; MoreHealth: Consultancy; Acerta Pharma: Honoraria, Research Funding; Novartis: Research Funding; Juno: Research Funding; Dava Oncology: Honoraria; Pharmacyclics: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal