Abstract
Background: R/R DLBCL remains an area of unmet medical need and treatments with novel mechanisms of action are urgently needed. Germinal center DLBCLs depend on the histone methyltransferase EZH2 to perpetuate a less-differentiated state and EZH2 activating mutations may be oncogenic drivers in a subset of patients (pts). Inhibition of EZH2 reprograms abnormal cell growth, leading to cell death or differentiation, and subsequent tumor regression. Tazemetostat, a potent, selective, oral EZH2 inhibitor has shown antitumor activity in a phase 1 study that included DLBCL pts with mutated (mt) or wild type (wt) EZH2 tumors, which provides rationale for further investigation of its single agent activity. This open-label, multicenter phase 2 study is evaluating tazemetostat in pts with either mt or wt EZH2 R/R DLBCL or follicular lymphoma (Grade 1-3b); results of an interim analysis of DLBCL pts treated with tazemetostat, as monotherapy or in combination with prednisolone are presented.
Methods: Key inclusion criteria include: age ≥18 years, ≥2 prior treatment regimens, and measurable disease. Tazmetostat 800 mg is administered orally, twice daily (BID); prednisolone (40 mg/m2) on days 1 to 5 and days 15 to 19 in a 28-day cycle for 16 weeks. Response was assessed every 8 weeks using IWG-NHL assessment criteria (Cheson 2007). Tumor tissue was analyzed for EZH2 hot spot activating mutations (Y646X, A682G, A692V) using a cobas® EZH2 Mutation Test (Roche Molecular Systems; investigational use only). Hans algorithm was used to determine cell of origin. The primary endpoint is overall response rate (ORR). Secondary endpoints include progression-free survival (PFS), duration of response (DOR) and safety/tolerability.
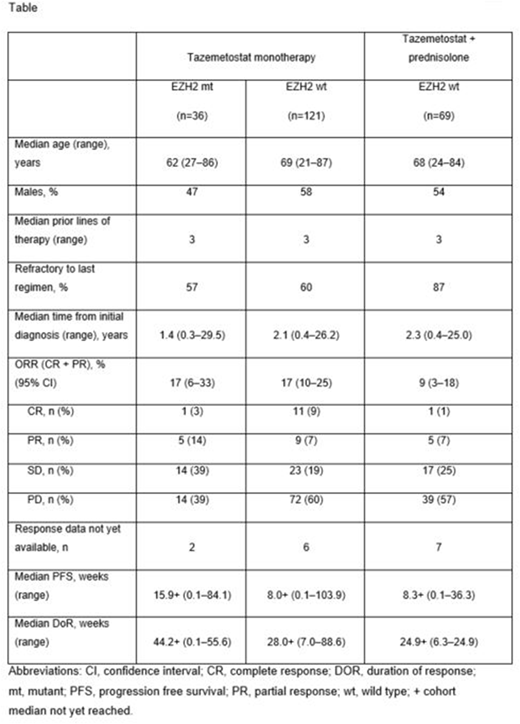
Results: As of May 1, 2018, interim phase 2 safety and activity data were summarized from 226 DLBCL pts (intent to treat analysis). Demographic and clinical activity information are provided in the table, including ORRs of 17% in both mt and wt arms and 9% in the prednisolone arm. Notably, DOR was substantially greater in the mt arm. Safety analysis showed that treatment-emergent adverse events (TEAE) leading to study drug discontinuation or withdrawal from study occurred in 12% of pts. Grade ≥3 treatment-related AEs were reported in 27% of pts. The most common (≥10%) TEAEs (all grades) were: thrombocytopenia (20%), nausea (17%), anemia (15%), neutropenia (15%), vomiting (15%), cough (14%), diarrhea (12%), fatigue (12%), pyrexia (12%), abdominal pain (11%) and asthenia (10%).
Conclusion: Tazemetostat was generally well tolerated at a dose of 800 mg BID, as monotherapy or in combination with prednisolone. In this difficult to treat, heavily pretreated, refractory patient population, clinical activity was observed in approximately 20% of monotherapy pts, regardless of mutational status, many of whom had received multiple prior lines of therapy. Tazemetostat in combination with prednisolone did not result in improved activity compared with tazemetostat monotherapy.
Ribrag:Servier, Pharmamar, Nanostring, Gilead, Infinity, BMS, MSD, Epizyme: Consultancy; Roche: Other: Travel, expenses, accommodation; ESAI: Honoraria, Research Funding. Morschhauser:Epizyme: Consultancy; Roche/Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees. McKay:Epizyme: Consultancy, Honoraria. Salles:Roche, Jannsen, Gilead, Celgene, Novartis, Amgen, BMS, Merck, Servier: Honoraria. Tilly:Roche: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Astra-Zeneca: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees. Cartron:Sanofi: Honoraria; Roche: Consultancy, Honoraria; Gilead Sciences: Honoraria; Janssen: Honoraria; Celgene: Consultancy, Honoraria. Gribben:Unum: Equity Ownership; Novartis: Honoraria; TG Therapeutics: Honoraria; Abbvie: Honoraria; Medical Research Council: Research Funding; Janssen: Honoraria, Research Funding; Roche: Honoraria; NIH: Research Funding; Wellcome Trust: Research Funding; Cancer Research UK: Research Funding; Pharmacyclics: Honoraria; Kite: Honoraria; Acerta Pharma: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Dickinson:GSK: Consultancy. Opat:Roche, Celgene, Mundipharma, Janssen: Honoraria; Roche, Celgene, Mundipharma, Janssen: Consultancy. Adib:Epizyme: Employment, Equity Ownership. Blakemore:Epizyme: Employment, Equity Ownership. Larus:Epizyme: Employment, Equity Ownership. Johnson:Zenyaku Kogyo: Other: Travel, accommodations, expenses; Eisai: Research Funding; Incyte: Consultancy; Genmab: Consultancy; Kite: Consultancy; Boeringher Ingelheim: Consultancy; Epizyme: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Honoraria; Novartis: Honoraria; Takeda: Honoraria, Travel, accommodations, expenses; Bristol-Myers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal