Abstract
Background: Currently, low molecular weight heparin is the guideline endorsed treatment of patients with cancer associated venous thromboembolism (VTE). While apixaban is approved for the treatment of acute VTE, there are limited data supporting its use in cancer patients.
Methods: Patients with cancer associated acute VTE were randomly assigned to receive either apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily or subcutaneous dalteparin (200 IU/kg for 1 month followed by 150 IU/kg once daily) for 6 months. The primary outcome was major bleeding. Secondary outcomes included VTE recurrence and a composite of major plus clinically relevant non-major bleeding.
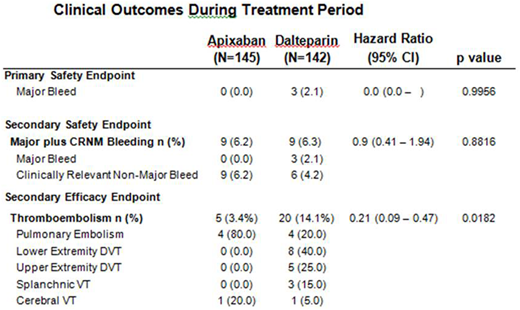
Results: Of the 300 patients who underwent randomization, 287 were included in the primary analysis. Of these, metastatic disease was present in 65.5% of subjects and 74% were receiving concurrent systemic cancer therapy. Colorectal, lung, pancreas, and breast cancers were the four most prevalent cancer types. Major bleeding occurred in 0 of the 142 patients (0%) in the apixaban group as compared with 3 of the 145 patients (2.1%) in the dalteparin group (p=0.9956). Recurrent VTE occurred in 5 patients (3.4%) in the apixaban group and 20 patients (14.1%) in the dalteparin group (difference in risk -10.7 percentage points) with a Hazard Ratio (HR) 0.26, (95% CI, 0.09 - 0.80, p = 0.0182). Major plus clinically relevant non-major bleeding were similar at 9% for both groups. There were no mortality differences comparing apixaban (15.9%) and dalteparin (10.6%) groups at 6 months (HR 1.36, 95% CI 0.79 - 2.35). Monthly quality of life surveys favored apixaban therapy for many measures including: concern for excess bruising, stress, irritation, burden of delivery, and overall satisfaction with anticoagulant therapy (p<0.05). Monthly bruising questionnaire favored apixaban at each interval (p<0.002).
Conclusions: Oral apixaban therapy was associated with very low rates of bleeding and significantly lower VTE recurrence with superior quality of life outcome measures compared to parenteral dalteparin in the treatment of cancer associated VTE. These data support the clinical utility of apixaban for the acute treatment of VTE in this patient population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal