Abstract
Introduction: The combined effect of cardiovascular (CV) risk and tyrosine kinase inhibitor (TKI) therapy may contribute to the incidence of CV events in patients (pts) with chronic phase chronic myeloid leukemia (CP-CML). CV events can affect the overall clinical outcomes and survival of CML patients, and hospitalization due to CV events is costly; analyzing the rates and risks of CV hospitalization in pts with CML receiving TKI therapy may help to inform treatment decisions and risk mitigation strategies and to minimize costs. SIMPLICITY (NCT01244750) is an ongoing observational study of CP-CML pts in routine clinical practice receiving first-line (1L) TKIs since 2010 in the US and Europe, exploring TKI use and management patterns in clinical practice. This analysis aims to evaluate rates of CV-related hospitalization and estimate related costs in pts treated with the second-generation TKIs dasatinib (DAS) and nilotinib (NIL).
Methods: CV hospitalizations were identified retrospectively from events reported by SIMPLICITY investigators over the course of treatment with DAS or NIL, from the start of treatment (any line of therapy) to 30 days after the end of that line of therapy, or prior to the start of a subsequent line of TKI therapy, whichever was sooner. Pts were followed for a maximum of five years. Owing to the observational nature of SIMPLICITY, patients were not matched between cohorts and adjustments for covariates were not made. Kaplan-Meier methods with the log-rank test were used to estimate time to first CV hospitalization. Statistical comparisons were made using t-tests and the Mann-Whitney U test for continuous variables and chi square for categorical variables.
Results: Overall, 825 pts received DAS (n=417) or NIL (n=408) as 1L therapy; 376 pts received DAS (n=214) or NIL (n=162) as second-line (2L) therapy; and 124 pts received DAS (n=63) or NIL (n=61) as third-line (3L) therapy. See table for patient baseline demographics and comorbidities. No significant differences were noted between the DAS and NIL cohorts. Median age was similar between the DAS and NIL groups at 56.2 and 54.1 years. At baseline, both groups had similar CV comorbidities. A significantly greater number of pts were treated in the US vs Europe (p=0.017).
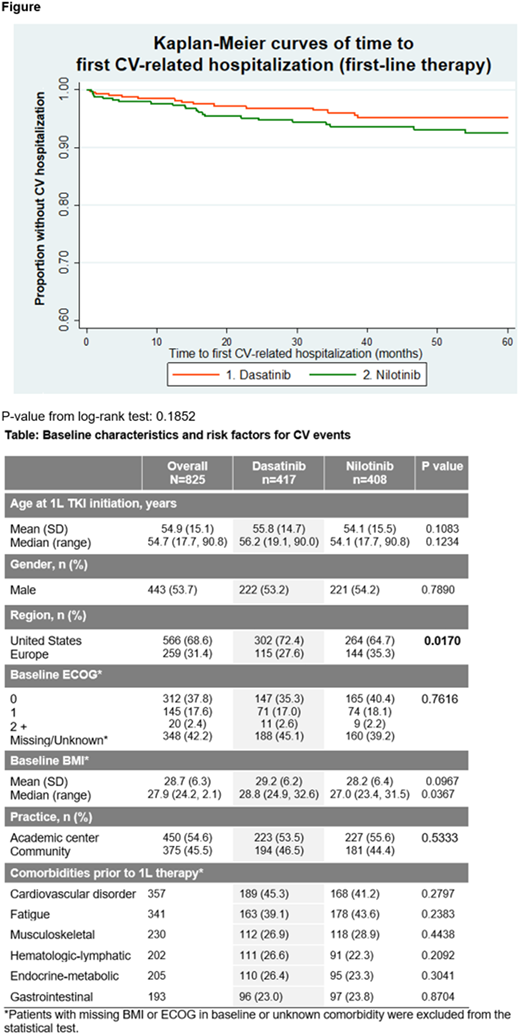
Investigators reported that, during 1L DAS and NIL therapy, 43% (n=357) of pts experienced a CV disorder: 45.3% (n=189) of DAS pts and 41.2% (n=168) of NIL pts (DAS vs NIL, p=0.28). Numbers of CV-related hospitalizations occurring during DAS and NIL therapy were 16 and 36, respectively, translating to 12.6 and 27.4 CV-related hospitalizations per 1000 pt years. Across 1L, 2L, and 3L (referred to here as 'all lines') of DAS and NIL therapy, 31 and 51 CV-related hospitalizations occurred, translating to 16.7 and 28.8 CV-related hospitalizations per 1000 pt years, respectively. The three most common CV-related reasons for hospitalization were congestive cardiac failure, atrial fibrillation and myocardial infarction. The figure shows Kaplan-Meier curves of time to first CV-related hospitalization for 1L therapy, for five years of follow-up. CV-related hospitalization at 5 years of follow-up was 5% for pts on 1L DAS, 7.5% for pts on 1L NIL, 5.7% for all lines of DAS and 8.5% for all lines of NIL. Durations of hospital stay related to CV disorders for pts on 1L DAS and 1L NIL were 68 days and 263 days, translating to 53.3 and 199.8 days in hospital per 1000 pt years. Durations of hospital stay related to CV disorders for pts on all lines of DAS and NIL were 107.4 days and 226.1 days, translating to 53.3 and 199.8 days in hospital per 1000 pt years. Incremental cost differences based on mean estimates will be presented.
Conclusions: CV disorders and related hospitalizations were frequently seen in patients with CP-CML in SIMPLICITY. In these patients, NIL was associated with a rate of CV hospitalization up to double that associated with DAS and lengthier hospital stay. Owing to the survival benefits conferred by TKIs, it is critical to minimize and manage complications that may arise in treated pts; potential for greater morbidity and healthcare cost should be considered when making decisions about TKIs to use in individual pts, particularly those with preexisting CV comorbidities.
Mauro:Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Research Funding. Chen:Bristol-Myers Squibb: Employment. Cortes:Novartis: Consultancy, Research Funding; Arog: Research Funding; Astellas Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding. Gambacorti-Passerini:Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy. Sen:ICON PLC: Employment. Gajavelli:Bristol-Myers Squibb: Employment. Davis:Bristol-Myers Squibb: Employment. Michallet:Octapharma: Membership on an entity's Board of Directors or advisory committees; Chugai: Consultancy; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal