Key Points
Viral infections upregulate IFITM3 in human platelets and MKs, triggering rapid antiviral immune responses.
MKs are effective immune cells that prevent virus infection in naive MKs but also limit infection in bystander hematopoietic stem cells.
Abstract
Evolving evidence indicates that platelets and megakaryocytes (MKs) have unexpected activities in inflammation and infection; whether viral infections upregulate biologically active, antiviral immune genes in platelets and MKs is unknown, however. We examined antiviral immune genes in these cells in dengue and influenza infections, viruses that are global public health threats. Using complementary biochemical, pharmacological, and genetic approaches, we examined the regulation and function of interferon-induced transmembrane protein 3 (IFITM3), an antiviral immune effector gene not previously studied in human platelets and MKs. IFITM3 was markedly upregulated in platelets isolated from patients during clinical influenza and dengue virus (DENV) infections. Lower IFITM3 expression in platelets correlated with increased illness severity and mortality in patients. Administering a live, attenuated DENV vaccine to healthy subjects significantly increased platelet IFITM3 expression. Infecting human MKs with DENV selectively increased type I interferons and IFITM3. Overexpression of IFITM3 in MKs was sufficient to prevent DENV infection. In naturally occurring, genetic loss-of-function studies, MKs from healthy subjects harboring a homozygous mutation in IFITM3 (rs12252-C, a common single-nucleotide polymorphism in areas of the world where DENV is endemic) were significantly more susceptible to DENV infection. DENV-induced MK secretion of interferons prevented infection of bystander MKs and hematopoietic stem cells. Thus, viral infections upregulate IFITM3 in human platelets and MKs, and IFITM3 expression is associated with adverse clinical outcomes. These observations establish, for the first time, that human MKs possess antiviral functions, preventing DENV infection of MKs and hematopoietic stem cells after local immune signaling.
Introduction
Dengue virus (DENV) is a positive-sense, single-stranded RNA virus; it is the most prevalent human arbovirus disease in the world (carried by Aedes mosquitos) and comprises 4 antigenically distinct serotypes (DENV types 1-4).1 DENVs infect hundreds of millions of people worldwide,2-4 causing substantial morbidity and mortality, and the prevalence of dengue is increasing. Dengue causes a spectrum of clinical manifestations, including vascular leakage, thrombocytopenia, and hemorrhage. In cases of dengue hemorrhagic fever, thrombocytopenia can be severe and cause life-threatening bleeding.1,2 Aedes mosquitoes are now commonly identified in southern parts of the United States, and the US population generally lacks immunity to dengue.2,5 Unfortunately, there are currently no vaccines or antiviral therapies approved by the US Food and Drug Administration for dengue.
Platelets are numerous and centrally positioned in the vasculature for immunosurveillance.6-9 Platelets possess a broad array of receptors, including Toll-like receptors (a key characteristic of innate immune cells9-11 ), and interact with other immune cells, including dendritic cells, lymphocytes, and myeloid leukocytes.8 Through these receptors and associated pathways, platelets sense and clear invading bacteria.12,13 Nevertheless, whether platelets and their progenitor cell, the megakaryocyte (MK), possess direct antiviral immune activities has not previously been reported.
We hypothesized that human viral infections (dengue and influenza) would upregulate potent antiviral immune genes in platelets and MKs, enhancing host responses to limit viral infection. We used next-generation RNA-sequencing (RNA-seq), followed by molecular, pharmacological, and genetic functional validation, to interrogate the transcriptome and proteome of isolated platelets from virally infected patients, during vaccine challenge trials and during in vitro infection of human MKs.
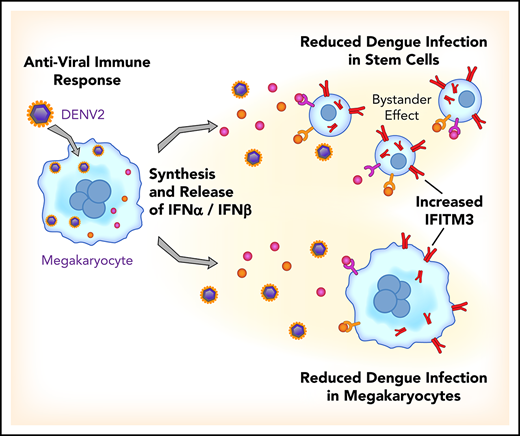
We identified that the antiviral immune protein interferon-induced transmembrane protein 3 (IFITM3) is significantly upregulated in platelets isolated from acutely infected patients with DENV or influenza and after administration of a live, attenuated DENV vaccine to healthy subjects. In patients with viral infections, lower platelet IFITM3 expression was associated with greater illness severity and mortality. Infecting human MKs with DENV resulted in a type I interferon (IFN) response and upregulation of IFITM3, similar to findings in patients with viral infections or receiving a viral challenge. The selective overexpression of IFITM3 in MKs was sufficient to significantly limit DENV infection. In genetic, loss-of-function studies, human MKs harboring a mutation in IFITM3 (rs12252-C) were significantly more susceptible to DENV infection. When MKs were exposed to DENV, viral infection was reduced not only in MKs but also in bystander hematopoietic stem cells (HSCs).
Thus, our findings show that during viral infections and human vaccine challenges with a live virus, the antiviral effector protein IFITM3 is upregulated in human MKs and platelets. Through upregulation of IFITM3, MKs acquire potent immune activities that limit cellular infection. These observations highlight the previously unrecognized antiviral function of MKs, thereby elucidating a completely new role for MKs during human viral infections.
Methods
The supplemental Methods (available on the Blood Web site) provide additional details.
Study design
All subjects provided written informed consent, and all study protocols were approved by an institutional review board. Patients with acute, primary, or secondary dengue infection (N = 29) were recruited from 2 sites: (1) the San Pablo Hospital in Bayamón, San Juan, Puerto Rico; and (2) the Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz in Rio de Janeiro, Brazil. DENV infection was confirmed serologically and/or molecularly by using real-time polymerase chain reaction (RT-PCR), in accordance with current standards. Patients (N = 24) with influenza A/H1N1 infection were recruited from 1 of 3 academic medical centers in Salt Lake City, Utah. Additional information on each study cohort is found in the supplemental Methods.
The dengue vaccine study was performed under an investigational new drug application reviewed by the US Food and Drug Administration. Written informed consent was obtained in accordance with federal and international regulations (21 CFR 50, ICH E6) with study subjects enrolled under study protocol CIR 323 (www.clinicaltrials.gov, NCT03416036). The live attenuated dengue vaccine (TV003) contains all 4 dengue serotypes (DENV 1-4) in a tetravalent admixture and was generated as previously described.14 Subjects were randomly selected to receive 0.5 mL of vaccine or placebo, administered subcutaneously on day 0. Platelets were isolated on day 0 and day 14, and IFITM3 expression was examined by using quantitative RT-PCR. All assays were performed before unblinding.
For the influenza vaccine studies, healthy adults were recruited from the University of Utah during the 2012 to 2013 influenza season. Platelets were isolated at day 0, immediately before administration of the influenza vaccine, and again 14 days after the influenza vaccine was administered. RNA was isolated and IFITM3 expression was measured by using quantitative RT-PCR.
Cell isolation, differentiation, and nomenclature
CD45 leukocyte-depleted human platelets were isolated as previously described15,16 from whole blood from dengue-infected patients and from healthy control subjects. The CD34+ cells were differentiated as previously described.17 DENV serotype 2 (DENV2) strain 16881 was propagated in C6/36 Aedes albopictus mosquito cells or, in some experiments, rhesus monkey kidney epithelial cells (LLC-MK2). CD34+-derived MKs were incubated on culture days 11 to 12 with DENV2 at a multiplicity of infection (MOI) of 1.0 for 18 hours at 37°C in a 5% carbon dioxide atmosphere. In some experiments, CD34+-derived MKs were pretreated with IFNs for up to 18 hours at various concentrations as indicated.
RNA preparation and sequencing
RNA-seq and analysis were performed on total RNA extracted from platelets isolated from confirmed, dengue-infected patients from Brazil and age-, sex-, and race-matched healthy subjects recruited from the same location. We also performed RNA-seq on a second cohort of confirmed, prospectively studied, dengue-infected patients from Puerto Rico and healthy control subjects recruited from the same location. Sequence reads were processed by the University of Utah Bioinformatics Core. FASTQ sequence reads were aligned with Novocraft’s Novo alignment program (Novocraft Technologies, Selangor, Malaysia), as previously described.18-21 RNA-seq raw data files are accessible through the National Center for Biotechnology Information’s Sequence Read Archive under bioproject identification numbers PRJNA517714 (SAMN10841493-SAMN10841506: Dengue Platelets, Dengue Healthy Control Platelets, H1N1 Platelets, Mock Treated, and Infected Megakaryocytes) and PRJNA397446 (SAMN07457428, SAMN07457429, and SAMN07457432: H1N1 Healthy Control Platelets).
RT-PCR analysis
For messenger RNA (mRNA) quantitative RT-PCR, isolated RNA was reverse transcribed as previously described.13,20,21 mRNA was normalized to the housekeeping transcripts glyceraldehyde-3-phosphate dehydrogenase (GADPH), β tubulin (TUBB), or β2-microglobulin (B2M) and quantified by using the 2−ΔΔCT method.22
Microscopy
High-resolution, confocal reflection microscopy was performed by using an Olympus IX81, FV300 (Olympus, Melville, NY) confocal-scanning microscope equipped with a 60×/1.42 NA oil objective for viewing platelets and MKs. Monochrome 16-bit images were further analyzed and changes quantified by using Adobe Photoshop CS6, ImageJ software (National Institutes of Health), and CellProfiler.21
MEG-01 transfection and dengue infection
The pZIP-mEF1a vector (shERWOOD-UltramiR lentiviral short hairpin RNA [shRNA]) was purchased from transOMICS Technologies (Huntsville, AL). IFITM3 was cloned into the pZIP empty vector by using single blunt-end ligation. MEG-01s were subjected to dengue infection in the presence of antibody against dengue at subneutralizing concentrations as previously described.23 Briefly, MEG-01s were infected with DENV (MOI 1) for 2 hours at 37°C. Cells were then washed (4 times) to remove residual free virus, and growth medium was replaced. Cells were incubated for 96 hours at 37°C before cell harvest to determine level of dengue infection. For shRNA knockdown of IFITM3, MEG-01s were transfected with SMARTvector Human Lentiviral IFITM3 shRNA or control vector (Dharmacon, Lafayette, CO) with a murine EF1a promoter and a TurboRFP marker (Addgene, Watertown, MA). After transfection, MEG-01s were flow sorted by RFP to enrich for sH-IFITM3 or control vector cells. MEG-01s were stimulated with 500 U/mL IFNα for 24 hours before proceeding with dengue infection as described earlier.
Statistical analyses
For all analyses, continuous variables were assessed for normality with skewness and kurtosis tests. Summary statistics were used to describe the study cohort, and clinical variables are expressed as the mean ± standard deviation or as a number and percentage. Parametric 2-tailed Student t tests or analysis of variance was used for continuous variables, and the χ2 and Fisher’s exact test were used for categorical variables. Statistical analyses were performed by using GraphPad Prism version 7 software (GraphPad Software, La Jolla, CA). A 2-tailed P < .05 was considered statistically significant.
Results
Dengue study cohort
Dengue-infected patients were well matched to the healthy subjects (supplemental Table 1). Approximately 59% of dengue-infected patients had mild disease, and 41% had dengue with warning signs or severe dengue infection, as defined according to World Health Organization guidelines. Dengue-infected patients were thrombocytopenic, as is common during dengue infection. Hemorrhagic manifestations, consistent with dengue hemorrhagic fever, were identified in ∼25% of patients with dengue. Primary dengue infection was more common than secondary dengue infection (69% vs 31%; P < .05). Slightly more than one-half of dengue-infected patients (15 of 29 [52%]) were treated as outpatients, and the remainder were hospitalized for treatment. The mean ± standard deviation length of stay for patients hospitalized was 2.7 ± 1.5 days. None of the dengue-infected patients died within 30 days of study enrollment. DENV was detected by using PCR in patients diagnosed with dengue infection (not shown).
The human platelet transcriptome is altered during dengue infection
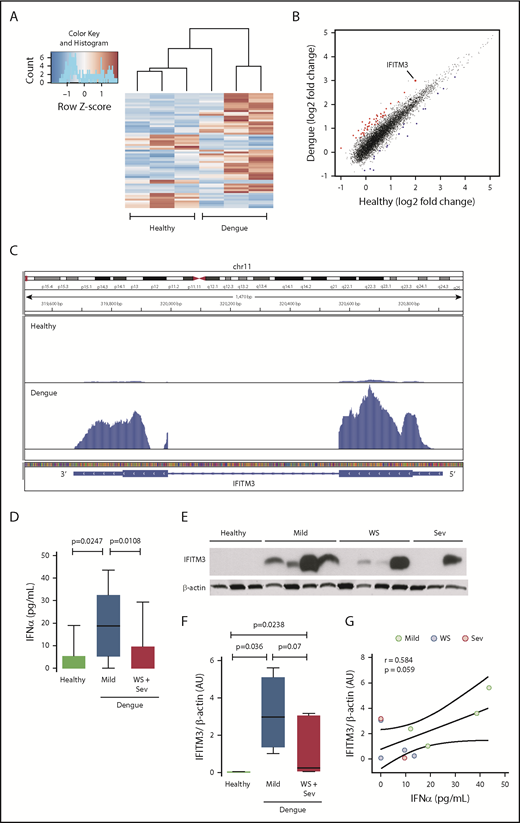
To determine if the platelet transcriptome is altered in patients during dengue infection, we performed RNA-seq on RNA isolated from highly purified platelets from patients infected with DENV in Brazil and matched healthy subjects recruited from the same Brazilian locale. Differential expression analyses identified 777 transcripts at a nominal significance (P < .05). Of these, 61 transcripts retained significance after stringent adjustment for multiple comparisons (false discovery rate, P < .05); 43 transcripts were upregulated, and 18 were downregulated (Figure 1A; supplemental Table 2). Of the significantly differentially expressed transcripts in platelets during dengue infection, a gene with highly increased expression was FITM3 (Figure 1B-C).
The human platelet transcriptome is altered during dengue infection. Platelets were isolated from patients with confirmed dengue infection or matched healthy control subjects. Purified platelets were isolated as described in “Methods.” (A) Heat map of significantly differentially expressed (adjusted P < .05) transcripts in dengue-infected patients (n = 3) and healthy control subjects (n = 3). Red indicates significantly enriched transcripts, and blue indicates significantly repressed transcripts. (B) Scatter plot with significantly enriched (red) and repressed (blue) transcripts. IFITM3 is indicated (enlarged dot). (C) Representative Integrative Genomics Viewer browser image of IFITM3 mRNA expression in a dengue-infected patient (bottom) or healthy control subject (top). The vertical axis represents the relative amount of mRNA, and the horizontal axis shows the introns (thin lines) and exons (thick lines). (D-G) Whole blood samples were drawn from patients with mild dengue infection (mild, n = 4), dengue-infected patients with warning signs (WS) or severe infection (Sev) (WS + Sev, n = 7), or healthy matched control subjects (n = 3). (D) Plasma levels of IFNα in healthy subjects (healthy) and in patients with mild dengue infection or severe dengue with WS. (E-F) Immunoblot and densitometric quantification of IFITM3 and β-actin expression in platelets isolated from healthy subjects (n = 3) and dengue-infected patients (n = 11) with mild, WS, or severe infection. The immunoblot results shown from healthy control subjects are representative of >30 individuals. (G) Plasma IFNα levels were plotted against IFITM3 protein expression in platelets from patients with dengue. Linear regression (± 95% confidence interval) was traced according to the distribution of the dots.
The human platelet transcriptome is altered during dengue infection. Platelets were isolated from patients with confirmed dengue infection or matched healthy control subjects. Purified platelets were isolated as described in “Methods.” (A) Heat map of significantly differentially expressed (adjusted P < .05) transcripts in dengue-infected patients (n = 3) and healthy control subjects (n = 3). Red indicates significantly enriched transcripts, and blue indicates significantly repressed transcripts. (B) Scatter plot with significantly enriched (red) and repressed (blue) transcripts. IFITM3 is indicated (enlarged dot). (C) Representative Integrative Genomics Viewer browser image of IFITM3 mRNA expression in a dengue-infected patient (bottom) or healthy control subject (top). The vertical axis represents the relative amount of mRNA, and the horizontal axis shows the introns (thin lines) and exons (thick lines). (D-G) Whole blood samples were drawn from patients with mild dengue infection (mild, n = 4), dengue-infected patients with warning signs (WS) or severe infection (Sev) (WS + Sev, n = 7), or healthy matched control subjects (n = 3). (D) Plasma levels of IFNα in healthy subjects (healthy) and in patients with mild dengue infection or severe dengue with WS. (E-F) Immunoblot and densitometric quantification of IFITM3 and β-actin expression in platelets isolated from healthy subjects (n = 3) and dengue-infected patients (n = 11) with mild, WS, or severe infection. The immunoblot results shown from healthy control subjects are representative of >30 individuals. (G) Plasma IFNα levels were plotted against IFITM3 protein expression in platelets from patients with dengue. Linear regression (± 95% confidence interval) was traced according to the distribution of the dots.
IFITM3 is upregulated in platelets from dengue-infected patients
Because IFITM3 is an IFN-sensitive gene, we questioned whether systemic IFN levels are increased in patients with dengue infection. Plasma levels of IFNα, a type I IFN, were significantly increased in dengue-infected patients compared with matched healthy subjects (Figure 1D). Intriguingly, IFNα levels were significantly lower in patients with severe dengue infection or dengue infection with warning signs, compared with patients with mild dengue infection. Plasma levels of IFNγ were also significantly elevated in subjects with mild and severe dengue infection (supplemental Figure 1A).
Because the transcript for IFITM3 was upregulated in platelets during dengue infection (Figure 1B-C), we next determined whether protein expression in platelets was coordinately increased. IFITM3 protein was generally undetectable in platelets isolated from healthy control subjects (Figure 1E). In contrast, IFITM3 protein was induced in platelets isolated from the majority of patients with dengue (Figure 1E-F). IFITM3 protein expression was lower in patients with more severe dengue infection, consistent with plasma IFNα levels (Figure 1D). IFITM3 protein expression in platelets correlated positively with systemic IFNα levels in dengue-infected patients (Figure 1G). Increased platelet IFITM3 expression in dengue was confirmed in a second, independent group of dengue-infected patients recruited from Puerto Rico, showing the reproducibility of our findings across 2 distinct patient groups (supplemental Figure 2).
IFITM3 is increased during human influenza infection
Influenza is one of the most common causes of respiratory illness in adults worldwide. With severe outbreaks of influenza infection, particularly of the influenza A/H1N1 strain, there is substantial morbidity and mortality. In nucleated cells, IFITM3 also controls influenza viral replication.24-26 Although influenza virus primarily infects the lungs, influenza infection is associated with platelet activation and a systemic IFN response.27,28 Therefore, we questioned if IFITM3 is induced in platelets during human influenza infection, similar to our observations in patients infected with dengue.
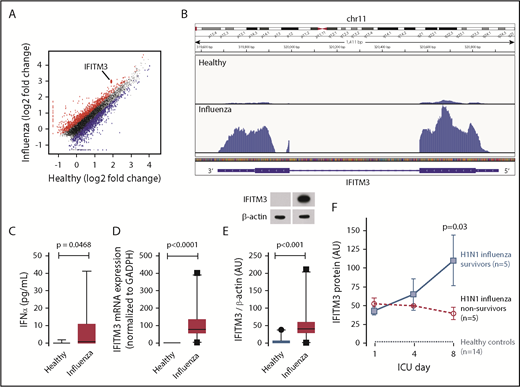
We prospectively recruited patients with primary influenza A/H1N1 and well-matched healthy subjects (supplemental Table 3). RNA-seq was first performed to determine whether both blood-borne (dengue) and pulmonary viral infections alter the human platelet transcriptome. Consistent with our findings during dengue infection (Figure 1A), the platelet transcriptome was substantially altered in patients with influenza A/H1N1 (Figure 2A), with ∼2900 transcripts that were either significantly upregulated or downregulated (adjusted false discovery rate, P < .05; supplemental Table 4). Of the significantly differentially expressed transcripts, only 37 were consistently altered in both dengue- and influenza-infected patients (supplemental Table 5). This finding suggests that changes in the platelet transcriptome are more specific to the underlying disease and not merely global activation responses. As with dengue, IFITM3 was among the top differentially expressed and enriched transcripts in platelets from patients with influenza (Figure 2A-B). Plasma levels of IFNα and IFNγ were also significantly increased in patients with influenza (Figure 2C; supplemental Figure 1B). We next validated IFITM3 mRNA and protein expression in a larger cohort (n = 31) of patients with influenza and matched healthy control subjects (n = 24). As shown in Figure 2D, platelet IFITM3 mRNA expression was ∼100-fold upregulated during influenza infection (P < .0001). Similar to findings during dengue infection, IFITM3 protein was generally not expressed in platelets from healthy control subjects but robustly induced during influenza infection (Figure 2E).
Platelet IFITM3 is increased in patients with H1N1/A influenza infection and correlates with influenza-related mortality. Whole blood samples were drawn from patients with confirmed H1N1/A influenza infection or healthy matched control subjects. Purified platelets were isolated as described in “Methods.” (A) Scatter plot with significantly enriched (red) and repressed (blue) platelet transcripts identified by using RNA-seq (adjusted P < .05). IFITM3 is indicated (enlarged dot). (B) Representative Integrative Genomics Viewer browser images of IFITM3 mRNA expression in a patient with H1N1/A influenza (bottom) or a healthy control subject (top). Representative of n = 3 patients with H1N1/A influenza and n = 3 healthy control subjects. (C) Plasma levels of IFNα in healthy control subjects and patients with H1N1/A influenza. (D) Validation with quantitative RT-PCR for IFITM3 mRNA was performed in platelets isolated from patients with H1N1/A influenza (n = 31) or healthy matched control subjects (n = 24). (E) Immunoblot (top) and densitometric quantification (bottom) of IFITM3 and β-actin expression in platelets from patients with H1N1/A influenza (n = 31) or healthy matched control subjects (n = 24). (F) In a subset of patients with H1N1/A influenza (n = 10), IFITM3 protein in platelets was measured longitudinally on ICU days 1, 4, and 8. Twenty-eight-day infection-related mortality was prospectively captured. Patients with H1N1/A influenza were divided into those who survived their influenza illness (survivors, n = 5) and those who died of their influenza illness before hospital discharge (nonsurvivors, n = 5). The dotted line on the bottom represents the average IFITM3 protein expression in platelets from healthy matched control subjects (n = 14).
Platelet IFITM3 is increased in patients with H1N1/A influenza infection and correlates with influenza-related mortality. Whole blood samples were drawn from patients with confirmed H1N1/A influenza infection or healthy matched control subjects. Purified platelets were isolated as described in “Methods.” (A) Scatter plot with significantly enriched (red) and repressed (blue) platelet transcripts identified by using RNA-seq (adjusted P < .05). IFITM3 is indicated (enlarged dot). (B) Representative Integrative Genomics Viewer browser images of IFITM3 mRNA expression in a patient with H1N1/A influenza (bottom) or a healthy control subject (top). Representative of n = 3 patients with H1N1/A influenza and n = 3 healthy control subjects. (C) Plasma levels of IFNα in healthy control subjects and patients with H1N1/A influenza. (D) Validation with quantitative RT-PCR for IFITM3 mRNA was performed in platelets isolated from patients with H1N1/A influenza (n = 31) or healthy matched control subjects (n = 24). (E) Immunoblot (top) and densitometric quantification (bottom) of IFITM3 and β-actin expression in platelets from patients with H1N1/A influenza (n = 31) or healthy matched control subjects (n = 24). (F) In a subset of patients with H1N1/A influenza (n = 10), IFITM3 protein in platelets was measured longitudinally on ICU days 1, 4, and 8. Twenty-eight-day infection-related mortality was prospectively captured. Patients with H1N1/A influenza were divided into those who survived their influenza illness (survivors, n = 5) and those who died of their influenza illness before hospital discharge (nonsurvivors, n = 5). The dotted line on the bottom represents the average IFITM3 protein expression in platelets from healthy matched control subjects (n = 14).
To begin to understand the kinetics of platelet IFITM3 expression during viral infection and associations with clinical outcomes, we longitudinally followed up a subset of patients with influenza (n = 10) during their hospitalization. Platelets from these patients were isolated upon intensive care unit (ICU) admission (day 1) and subsequently on ICU days 4 and 8. Over this 8-day period (which allows for near full platelet turnover), platelet IFITM3 protein expression continued to increase in patients who survived to ICU discharge. In comparison, platelet IFITM3 failed to increase in nonsurviving patients with influenza (Figure 2F).
Dengue vaccination in healthy subjects increases platelet IFITM3
The effects of vaccination on platelet immune genes have never been examined, to the best of our knowledge. We next questioned whether TV003, a live, attenuated dengue vaccine being studied in clinical trials,14 upregulated IFITM3 expression in platelets. For comparison, platelet IFITM3 expression in healthy subjects administered the inactivated seasonal trivalent influenza vaccine was also examined. Appropriate A/H1N1 responses to the influenza vaccine were confirmed by using the hemagglutination inhibition assay (not shown).29 Live dengue vaccination, but not inactivated influenza vaccination, significantly increased platelet IFITM3 expression (Figure 3A-D).
Administration of a live dengue vaccine to healthy subjects upregulates platelet IFITM3. (A) Schematic of the dengue vaccination clinical trial. After providing informed consent, healthy subjects were randomized to receive either a live, attenuated dengue vaccine (TV003, n = 17) or placebo injection (Placebo, n = 8) in a 2:1 randomization scheme. Investigators were blinded to study group assignment. Immediately before injection (day 0 [D0]) and again 14 days after injection (D14), whole blood samples were drawn from subjects. Purified platelets were isolated as described in “Methods.” (B) IFITM3 expression was measured by using quantitative RT-PCR in isolated platelets. The graph shows the change in IFITM3 expression on D14, compared with D0 levels. (C) Schematic of the influenza vaccination study. Whole blood samples were drawn from healthy subjects (n = 8) immediately before (D0) receiving the 2012 to 2013 inactivated influenza vaccine and again 14 days (D14) after vaccination. Purified platelets were isolated as described in “Methods.” (D) IFITM3 expression was measured by using quantitative RT-PCR in isolated platelets.
Administration of a live dengue vaccine to healthy subjects upregulates platelet IFITM3. (A) Schematic of the dengue vaccination clinical trial. After providing informed consent, healthy subjects were randomized to receive either a live, attenuated dengue vaccine (TV003, n = 17) or placebo injection (Placebo, n = 8) in a 2:1 randomization scheme. Investigators were blinded to study group assignment. Immediately before injection (day 0 [D0]) and again 14 days after injection (D14), whole blood samples were drawn from subjects. Purified platelets were isolated as described in “Methods.” (B) IFITM3 expression was measured by using quantitative RT-PCR in isolated platelets. The graph shows the change in IFITM3 expression on D14, compared with D0 levels. (C) Schematic of the influenza vaccination study. Whole blood samples were drawn from healthy subjects (n = 8) immediately before (D0) receiving the 2012 to 2013 inactivated influenza vaccine and again 14 days (D14) after vaccination. Purified platelets were isolated as described in “Methods.” (D) IFITM3 expression was measured by using quantitative RT-PCR in isolated platelets.
DENV infection upregulates antiviral immune genes in human MKs
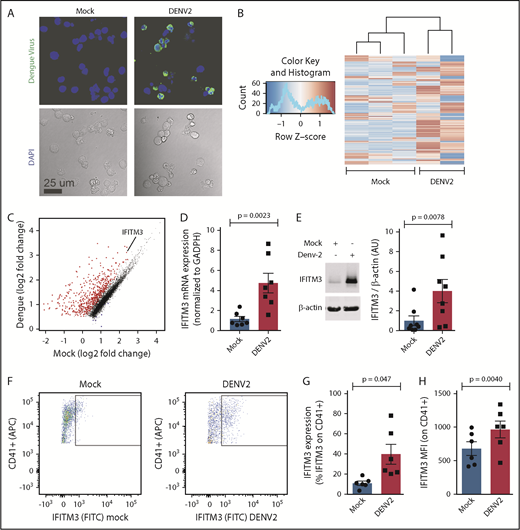
DENV invades the bloodstream and is found within the bone marrow niche, providing opportunities for directly infecting and/or signaling to MKs.30-33 Thus, we next hypothesized that exposure to pathogenic DENV induces immune gene expression in human MKs. To test this theory, we infected human CD34+-derived MKs with DENV2, a common cause of infection worldwide. Human MKs were permissive to DENV2 infection (Figure 4A). DENV2 also infected human platelets (not shown), consistent with previous studies.34 As we observed in platelets isolated from dengue-infected patients (Figure 1), DENV2 infection markedly altered the MK transcriptome (Figure 4B-C). Also consistent with findings in platelets from patients with dengue (supplemental Table 6), the antiviral immune gene IFITM3 was one of the highest enriched transcripts altered in MKs upon dengue exposure in vitro. mRNA and protein validation studies confirmed that DENV significantly upregulated IFITM3 in human MKs (Figure 4D-H). Intracellular localization experiments indicate that IFITM3 in MKs was primarily located in late-stage endosomes and early lysosomes (supplemental Figure 3).
DENV infects human MKs and upregulates IFITM3. (A) CD34+-derived MKs (culture day 12) were incubated (t = 18 hours) with mock virus (mock) or DENV2 (MOI 1.0). After incubation, MKs were stained by immunocytochemistry against DENV2 antigen (green). Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Data shown in this figure are from 5 to 6 independent experiments per group. (B) Heat map showing differential levels of expression in CD34+-derived MKs (culture day 12) incubated (t = 18 hours) with mock or DENV2 (MOI 1.0). (C) Scatter plot showing significantly (adjusted P < .05) enriched (red) and repressed (blue) transcripts in dengue-infected MKs compared with mock-infected MKs. IFITM3 is indicated. (D-E) CD34+-derived MKs (culture day 12) were incubated (t = 18 hours) with mock or DENV2 (MOI 1.0). IFITM3 was measured by using quantitative RT-PCR (D) and immunoblot densitometry (E). Dengue-infected CD34+-derived MKs (and mock-infected control MKs) were stained with antibodies against CD41 (a mature MK marker) and IFITM3. Intracellular IFITM3 expression in CD41+ mature, human MKs was quantified by using flow cytometry. (F) Representative histograms. Quantification is shown in percent positive cells (G) and mean fluorescence intensity (MFI; H), n = 6.
DENV infects human MKs and upregulates IFITM3. (A) CD34+-derived MKs (culture day 12) were incubated (t = 18 hours) with mock virus (mock) or DENV2 (MOI 1.0). After incubation, MKs were stained by immunocytochemistry against DENV2 antigen (green). Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Data shown in this figure are from 5 to 6 independent experiments per group. (B) Heat map showing differential levels of expression in CD34+-derived MKs (culture day 12) incubated (t = 18 hours) with mock or DENV2 (MOI 1.0). (C) Scatter plot showing significantly (adjusted P < .05) enriched (red) and repressed (blue) transcripts in dengue-infected MKs compared with mock-infected MKs. IFITM3 is indicated. (D-E) CD34+-derived MKs (culture day 12) were incubated (t = 18 hours) with mock or DENV2 (MOI 1.0). IFITM3 was measured by using quantitative RT-PCR (D) and immunoblot densitometry (E). Dengue-infected CD34+-derived MKs (and mock-infected control MKs) were stained with antibodies against CD41 (a mature MK marker) and IFITM3. Intracellular IFITM3 expression in CD41+ mature, human MKs was quantified by using flow cytometry. (F) Representative histograms. Quantification is shown in percent positive cells (G) and mean fluorescence intensity (MFI; H), n = 6.
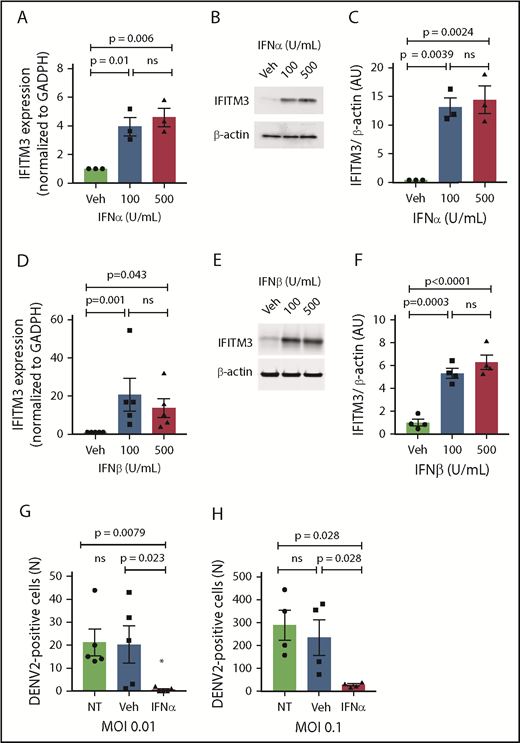
Interferons upregulate IFITM3 in human MKs
We next examined whether type I IFNs, which are increased during dengue and influenza infections (Figures 1D and 2C), regulate the transcription and translation of IFITM3 in human MKs. Stimulating MKs with either IFNα or IFNβ,35,36 at concentrations similar to systemic plasma levels observed in dengue-infected patients, robustly upregulated IFITM3 (Figure 5A-F). A type II IFN (IFNγ) also increased IFITM3 expression (supplemental Figure 4). Preconditioning MKs with IFNα significantly reduced dengue infection (Figure 5G-H).
Type I IFNs upregulate IFITM3 and block dengue infection of human MKs. (A-F) Human, CD34+-derived MKs (culture day 12) were stimulated with IFNα or IFNβ at various concentrations or vehicle control (Veh) for 18 hours. IFITM3 mRNA expression (A) or protein expression (B) in IFNα-stimulated MKs was determined by using quantitative RT-PCR and immunoblot. (C) Immunoblots shown in panel B were quantified by densitometry. (D) IFITM3 mRNA expression in IFNβ-stimulated MKs was determined by using quantitative RT-PCR. (E-F) IFITM3 protein expression in IFNβ-stimulated MKs was measured by immunoblot and quantified by densitometry. (G-H) CD34+ MKs (culture day 11) were left alone (NT) or preconditioned with IFNα (100 U/mL) or Veh (phosphate-buffered saline) for 18 hours. CD34+ MKs were then infected with DENV2 at an MOI of 0.01 (G) or an MOI of 0.1 (H) for 18 hours. Dengue infection was determined by measuring the titers of virus released by MKs in LLC-MK2 cells. Data are from >4 to 6 independent experiments per group.
Type I IFNs upregulate IFITM3 and block dengue infection of human MKs. (A-F) Human, CD34+-derived MKs (culture day 12) were stimulated with IFNα or IFNβ at various concentrations or vehicle control (Veh) for 18 hours. IFITM3 mRNA expression (A) or protein expression (B) in IFNα-stimulated MKs was determined by using quantitative RT-PCR and immunoblot. (C) Immunoblots shown in panel B were quantified by densitometry. (D) IFITM3 mRNA expression in IFNβ-stimulated MKs was determined by using quantitative RT-PCR. (E-F) IFITM3 protein expression in IFNβ-stimulated MKs was measured by immunoblot and quantified by densitometry. (G-H) CD34+ MKs (culture day 11) were left alone (NT) or preconditioned with IFNα (100 U/mL) or Veh (phosphate-buffered saline) for 18 hours. CD34+ MKs were then infected with DENV2 at an MOI of 0.01 (G) or an MOI of 0.1 (H) for 18 hours. Dengue infection was determined by measuring the titers of virus released by MKs in LLC-MK2 cells. Data are from >4 to 6 independent experiments per group.
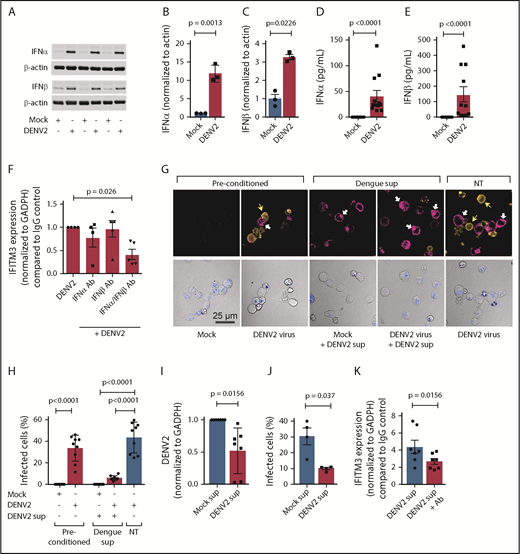
IFITM3 in MKs restricts DENV infection
We observed that when MKs are infected with DENV, IFITM3 is upregulated in some, but not all, cells (supplemental Figure 5A). MKs were permissive to dengue infection, shown by measuring viral titers from infected MKs by using a plaque assay (supplemental Figure 5B). Dengue infection in MKs was also confirmed in parallel experiments using microscopy, to visually identify infected cells, and quantitative RT-PCR, to measure the amount of DENV RNA (supplemental Figure 5C-D). Of even greater interest to us, MKs expressing IFITM3 generally seemed to be protected from DENV infection; in contrast, MKs not expressing IFITM3 protein seemed to be permissive to dengue infection. This scenario was reminiscent of cellular immune responses regulated by extracellular paracrine signaling. This finding led us to hypothesize that when MKs sense DENV, they actively produce and secrete IFNs that upregulate IFITM3 in surrounding cells, thereby helping limit viral infection.
We first examined whether dengue infection upregulates type I IFNs in human MKs. DENV significantly increased the expression of IFNA2 and IFNB1 mRNA expression in MKs (supplemental Figure 6A-B). This finding was accompanied by significant increases in the synthesis of IFNα and IFNβ protein in MKs (Figure 6A-C). In contrast, the expression of IFNγ, a type II IFN, was not increased in MKs during dengue infection (supplemental Figure 6C). DENV also increased the secretion of IFNα and IFNβ by MKs (Figure 6D-E; supplemental Figure 6D). Inhibiting either IFNα or IFNβ alone during dengue infection did not significantly affect IFITM3 expression. However, inhibiting both IFNα and IFNβ in combination significantly reduced IFITM3 expression in MKs (Figure 6F).
MKs exposed to dengue release type I IFNs to induce IFITM3 and limit viral infection. (A-E) CD34+-derived human MKs were infected with DENV2 (MOI 1.0; culture day 12; t = 18 hours) or mock control. (A-C) Type I IFN (IFNα and IFNβ) protein expression was measured according to immunoblot in MK cell lysates and quantified according to densitometry. Immunoblots show n = 3 independent biological replicates. (D-E) Supernatants from MKs infected with DENV2 were also measured for IFNα and IFNβ by using an enzyme-linked immunosorbent assay (n = 10-11). (F) MKs were infected with DENV2 in the presence of blocking antibodies specifically against IFNα (1 μg/mL), IFNβ (1 μg/mL), or both or immunoglobulin G (IgG) controls. IFITM3 mRNA expression in MKs was measured by using quantitative RT-PCR (qRT-PCR) (n = 4-5). (G) MKs were preconditioned with DENV2 (MOI 0.1) or mock virus control (mock) on culture day 10 (t = 18 hours). After 18 hours, supernatants were harvested from DENV2 or mock preconditioned MKs. Freshly cultured MKs were then treated with harvested supernatants from DENV2 preconditioned MKs (+DENV2 sup) or left alone (NT), and infected with DENV (DENV2 virus, MOI 1.0) or mock control (Mock). After 18 hours, dengue infection in MKs was determined by using immunocytochemistry with an antibody against DENV2 (orange, top row). In parallel, IFITM3 protein expression was examined (magenta, top row). Nuclei were stained by using TO-PRO-3 (blue) and shown in the bottom panels depicting the transmission channel. The white arrows point to IFITM3-positive cell bodies, and the yellow arrows highlight cells that stained positive for DENV2. Scale bars = 25 µm. (H) Images were quantified by using CellProfiler as described in “Methods.” The bar graph shows the percentage of DENV2-infected MKs. (I-J) Human HSCs were pretreated with harvested supernatants from mock (Mock sup) or dengue (DENV2 sup) infected MKs, under conditions described in (E). (I) DENV2 mRNA was measured by using qRT-PCR, and (J) the number of dengue-infected HSCs were quantified by microscopy. (K) HSCs were infected with DENV2 in the presence of blocking antibodies specifically against IFNα or IFNβ or both or IgG controls. IFITM3 mRNA expression in MKs was measured by using qRT-PCR (n = 7).
MKs exposed to dengue release type I IFNs to induce IFITM3 and limit viral infection. (A-E) CD34+-derived human MKs were infected with DENV2 (MOI 1.0; culture day 12; t = 18 hours) or mock control. (A-C) Type I IFN (IFNα and IFNβ) protein expression was measured according to immunoblot in MK cell lysates and quantified according to densitometry. Immunoblots show n = 3 independent biological replicates. (D-E) Supernatants from MKs infected with DENV2 were also measured for IFNα and IFNβ by using an enzyme-linked immunosorbent assay (n = 10-11). (F) MKs were infected with DENV2 in the presence of blocking antibodies specifically against IFNα (1 μg/mL), IFNβ (1 μg/mL), or both or immunoglobulin G (IgG) controls. IFITM3 mRNA expression in MKs was measured by using quantitative RT-PCR (qRT-PCR) (n = 4-5). (G) MKs were preconditioned with DENV2 (MOI 0.1) or mock virus control (mock) on culture day 10 (t = 18 hours). After 18 hours, supernatants were harvested from DENV2 or mock preconditioned MKs. Freshly cultured MKs were then treated with harvested supernatants from DENV2 preconditioned MKs (+DENV2 sup) or left alone (NT), and infected with DENV (DENV2 virus, MOI 1.0) or mock control (Mock). After 18 hours, dengue infection in MKs was determined by using immunocytochemistry with an antibody against DENV2 (orange, top row). In parallel, IFITM3 protein expression was examined (magenta, top row). Nuclei were stained by using TO-PRO-3 (blue) and shown in the bottom panels depicting the transmission channel. The white arrows point to IFITM3-positive cell bodies, and the yellow arrows highlight cells that stained positive for DENV2. Scale bars = 25 µm. (H) Images were quantified by using CellProfiler as described in “Methods.” The bar graph shows the percentage of DENV2-infected MKs. (I-J) Human HSCs were pretreated with harvested supernatants from mock (Mock sup) or dengue (DENV2 sup) infected MKs, under conditions described in (E). (I) DENV2 mRNA was measured by using qRT-PCR, and (J) the number of dengue-infected HSCs were quantified by microscopy. (K) HSCs were infected with DENV2 in the presence of blocking antibodies specifically against IFNα or IFNβ or both or IgG controls. IFITM3 mRNA expression in MKs was measured by using qRT-PCR (n = 7).
Because dengue increases IFN secretion by MKs, and IFNs restrict dengue infection in MKs (Figures 5 and 6), we next examined whether supernatants from dengue-infected MKs have direct antiviral immune functions. Dengue infected ∼35% to 40% of the MKs (Figure 6G-H; supplemental Figure 5A) and resulted in increased intracellular and surface IFITM3 expression on noninfected MKs (Figure 6G, far left 2 panels). Interestingly, isolated supernatants from preconditioned, dengue-infected human MKs upregulated IFITM3 (in the absence of new DENV infection) and significantly reduced dengue infection in naive MKs. This finding indicates that MKs secrete biologically active antiviral proteins that augment host immune responses. We next questioned whether MKs release antiviral proteins that also block DENV infection in human stem cells, as stem cells and MKs are both resident in close proximity in the bone marrow niche. Isolated supernatants from preconditioned, dengue-infected MKs significantly blocked dengue infection of primary CD34+ HSCs (Figure 6I-J). Similar to MKs, inhibition of both IFNα and IFNβ in supernatants from preconditioned, dengue-infected MKs reduced IFITM3 expression in HSCs, suggesting that type I IFNs in the supernatant regulate IFITM3 expression and subsequent dengue infection (Figure 6K).
To determine if IFITM3 in MK specifically prevents DENV infection, we next selectively overexpressed or deleted IFITM3 in a megakaryocytic cell line (MEG-01s). MEG-01s are a well-accepted and biologically relevant megakaryocytic cell line more amenable to genetic manipulation. We confirmed that IFITM3 mRNA and protein were endogenously upregulated in unstimulated pZIP-IFITM3 MEG-01s, indicating that overexpression of IFITM3 was successful (Figure 7A-B). Overexpression of IFITM3 in MEG-01s was sufficient to significantly reduce DENV infection in the absence of stimulation with IFNs (Figure 7C). We next examined if reducing IFITM3 expression resulted in increased dengue infection. Using shRNA targeting IFITM3, we observed that MEG-01s had reduced IFITM3 expression after IFNα treatment compared with control shRNA. To examine the functional consequence of reduced IFITM3 expression, both control and IFITM3 shRNA MEG-01s were infected with DENV. shRNA knockdown of IFITM3 rendered MKs significantly more susceptible to dengue infection (Figure 7D-F).
IFITM3 in MKs functions to limit dengue infection. MEG-01s were left alone (No pZIP), or transfected with an empty vector control (pZIP + EV) or an engineered vector containing IFITM3 (pZIP-IFITM3 [eg, IFITM3 overexpression]). IFITM3 mRNA (A) and IFITM3 protein (B) were determined by using quantitative RT-PCR (qRT-PCR) and immunoblot, respectively, to confirm IFITM3 overexpression in unstimulated MEG-01s after transfection. (C) pZIP-EV control or pZIP-IFITM3 overexpressing MEG-01s were infected with DENV2 (t = 96 hours; MOI 0.1) in the presence of subneutralizing concentrations of monoclonal antibodies against DENV2. Antiflavivirus antibody 4G2 (200 ng) was mixed with DENV2 (MOI 0.1) and incubated with MEG-01s. DENV2 mRNA was measured by using qRT-PCR. (D-E) MEG-01s were transfected with control shRNA (sh-NC) or an engineered vector containing shRNA against IFITM3 (sh-IFITM3). sh-NC and sh-IFITM3 MEG-01s were treated with IFNα or vehicle control, and IFITM3 protein was measured (n = 4). (F) sh-NC or sh-IFITM3 MEG-01s were infected with DENV2 (t = 96 hours; MOI 0.1) via antibody-dependent enhancement mode of infection with DENV2 as described earlier. DENV2 mRNA was measured by using qRT-PCR (n = 6). (G-H) A naturally occurring IFITM3 loss-of-function homozygous variant in human MKs increased susceptibility to dengue infection. (G) Representative Sanger sequencing of CD34+-derived human MKs harboring either the ancestral IFITM3 allele T (WT, left) or the IFITM3 loss-of-function single-nucleotide polymorphism variant rs12252T-C (IFITM3 variant, right). Red rectangles highlight the single nucleotide change in IFITM3 from a thymine (T, ancestral) to a cytosine (C, variant). (H) MKs harboring either ancestral IFITM3 or the loss-of-function IFITM3 variant (n = 4 different subjects per group) were infected with DENV2 (t = 96 hours; MOI 0.1). DENV2 mRNA was measured by using qRT-PCR and normalized to GAPDH.
IFITM3 in MKs functions to limit dengue infection. MEG-01s were left alone (No pZIP), or transfected with an empty vector control (pZIP + EV) or an engineered vector containing IFITM3 (pZIP-IFITM3 [eg, IFITM3 overexpression]). IFITM3 mRNA (A) and IFITM3 protein (B) were determined by using quantitative RT-PCR (qRT-PCR) and immunoblot, respectively, to confirm IFITM3 overexpression in unstimulated MEG-01s after transfection. (C) pZIP-EV control or pZIP-IFITM3 overexpressing MEG-01s were infected with DENV2 (t = 96 hours; MOI 0.1) in the presence of subneutralizing concentrations of monoclonal antibodies against DENV2. Antiflavivirus antibody 4G2 (200 ng) was mixed with DENV2 (MOI 0.1) and incubated with MEG-01s. DENV2 mRNA was measured by using qRT-PCR. (D-E) MEG-01s were transfected with control shRNA (sh-NC) or an engineered vector containing shRNA against IFITM3 (sh-IFITM3). sh-NC and sh-IFITM3 MEG-01s were treated with IFNα or vehicle control, and IFITM3 protein was measured (n = 4). (F) sh-NC or sh-IFITM3 MEG-01s were infected with DENV2 (t = 96 hours; MOI 0.1) via antibody-dependent enhancement mode of infection with DENV2 as described earlier. DENV2 mRNA was measured by using qRT-PCR (n = 6). (G-H) A naturally occurring IFITM3 loss-of-function homozygous variant in human MKs increased susceptibility to dengue infection. (G) Representative Sanger sequencing of CD34+-derived human MKs harboring either the ancestral IFITM3 allele T (WT, left) or the IFITM3 loss-of-function single-nucleotide polymorphism variant rs12252T-C (IFITM3 variant, right). Red rectangles highlight the single nucleotide change in IFITM3 from a thymine (T, ancestral) to a cytosine (C, variant). (H) MKs harboring either ancestral IFITM3 or the loss-of-function IFITM3 variant (n = 4 different subjects per group) were infected with DENV2 (t = 96 hours; MOI 0.1). DENV2 mRNA was measured by using qRT-PCR and normalized to GAPDH.
A human loss-of-function variant of IFITM3 increases dengue infection in MKs
A naturally occurring loss-of-function variant in IFITM3 (rs12252, Δ21 IFITM3) has been associated with an increased risk of severe influenza infection.25,37 Whether this mutation also increases susceptibility to dengue infection, in MKs or in any primary human cell, has never been examined, to our knowledge. Using Sanger sequencing, human MKs harboring this loss-of-function variant were identified. Human MKs harboring the IFITM3 variant were more susceptible to dengue infection compared with MKs without the IFITM3 variant (Figure 7G-H).
Discussion
Emerging evidence implicates platelets as having dynamic and versatile immunological functions in addition to their canonical roles in hemostasis.13,28,38-40 Platelets mediate host defense mechanisms against bacterial pathogens through a number of mechanisms, including the release of kinocidins and microbicidal proteins stored in α granules.38,41-43 It is believed that the molecular factors packaged in platelets are invested to them by their parent cell (the MK) during the process of platelet formation. A mass spectrometry–based investigation identified that the platelet proteome is altered during dengue infection.44 Nevertheless, changes in the platelet molecular signature during influenza infection, a virus not typically identified in the bloodstream or bone marrow, have not been studied. Moreover, whether viral infections upregulate antiviral immune genes in platelets and MKs, thereby rendering cells nonpermissive to infection, has not previously been established.
The present study found that the platelet transcriptome is dynamically altered during influenza and dengue viral infections. We found that type I IFNs (IFNα and IFNβ), but not IFNγ, were increased and secreted by MKs upon infection with DENV. This outcome is consistent with previous literature suggesting that dengue and influenza induce IFNα and IFNβ synthesis.45,46 We further identified that the IFN-sensitive viral restriction factor, IFITM3, is robustly induced both at the mRNA and protein level in human platelets and MKs during viral infections. Additional IFN responsive genes were upregulated during influenza and/or dengue infection, including IFITM1 and the IFN γ receptor 1 (IFNGR1) (supplemental Tables 2 and 4). However, our studies focused on IFITM3 because this gene was the only IFN-sensitive gene consistently and significantly upregulated in platelets during clinical dengue and influenza infection. Using a combination of pharmacological and genetic approaches, including MKs harboring a naturally occurring IFITM3 loss-of-function variant, we found that through regulated induction of IFITM3, MKs possess intrinsic antiviral immunity. Overexpression of IFITM3 was sufficient to reduce dengue infection of MKs through antibody-dependent enhancement, an important mode of secondary dengue infection involving virus uptake through Fcγ receptors.47 To our knowledge, this analysis is the first evidence that MKs directly restrict dengue infection. Moreover, this intrinsic antiviral response renders both MKs and HSCs nonpermissive to dengue infection, thereby showing that MKs limit dengue infection in bystander cells resident in the bone marrow niche. Although not a central focus of our study, these findings also confirm that MKs have functional type I and type II IFN receptors.48,49
Administration of a live attenuated dengue vaccine to healthy subjects, TV003, altered IFITM3 expression in platelets. However, an inactivated influenza vaccine did not change expression of platelet IFITM3. These findings have 2 implications. First, they suggest that vaccination may modify platelet gene expression, an intriguing possibility that may assist with future vaccine development efforts. Second, these data suggest live attenuated vaccines may alter functional responses in platelets, allowing them to play a critical, yet unrecognized, role in the interface between the innate and adaptive immunity. Future studies are necessary to elucidate if alterations in the platelet transcriptome after vaccination are virus specific or due to the nature of the vaccine (eg, live vs inactivated).
Although we observed increased IFITM3 in platelets isolated from patients with influenza and dengue infection, we chose to focus mechanistically on the role of IFITM3 in MKs during dengue infection. DENV infects bone marrow MKs in vitro and in vivo, resulting in decreased platelet production.30-32,50,51 Increased dengue copy number in platelets has been directly linked to platelet activation and the degree of thrombocytopenia.52 DENV also activates the inflammasome in platelets and promotes the synthesis of IL-1β, which may contribute to the increased vascular permeability common during dengue infection.53,54 Interestingly, mild dengue infection was seen more in patients with primary dengue infection, and it induced a more potent IFN response and increased IFITM3 expression in platelets compared with secondary dengue cases. This finding is consistent with previous studies, which reported increased IFNs in primary dengue infection due to high levels of viremia.36,55,56
Thrombocytopenia is common during viral infections such as dengue.31-33,50,52 Although still incompletely understood, thrombocytopenia during viral infections may be due in part to direct infection of MKs.31-33 DENV, hantavirus, and Junin virus can directly infect MKs, increasing apoptosis and suppressing MK maturation.57 These viruses also reduce surface expression of c-Mpl, the receptor for thrombopoietin.58 Because IFITM3 was found on the MK cell surface, we speculate that by preventing viral infection, IFITM3 may indirectly help prevent thrombocytopenia. Interestingly, MKs from healthy subjects that contain a single-nucleotide polymorphism in IFITM3 (rs12252) were more permissive to dengue infection. This variant, encoded by the rs12252(C) allele, is associated with loss of function of IFITM3 in some viral challenges, including influenza. The (C/C) genotype is enriched in Han Chinese populations and, although still somewhat controversial, has been associated with more severe influenza illness in these patients.25,37 Our data extend these studies by showing that MKs from individuals with the (C/C) genotype are also more permissive to dengue infection. Based on data from the 1000 Genomes Project, the frequency of this allele is low in European subjects (4%) but high in populations in which dengue is endemic, including Puerto Rico (11%), Africa (26%), and Asia (53%).
We also found that IFITM3 mRNA and protein are highly upregulated in platelets from patients acutely infected with influenza A/H1N1 virus. In vivo, IFITM3 deficiency increases susceptibility to influenza virus.25,26 Influenza primarily infects the lung, and autopsies of influenza-infected patients have revealed platelets located in extravascular spaces within the infected lungs.59-61 Furthermore, platelets also accumulate in bronchoalveolar lavage fluid obtained from the lungs of influenza-infected mice.28 Recent reports also indicate that MKs are located in the lung and increase in number during infection.62 These studies suggest that MKs and platelets may have opportunities to directly interact with influenza virus infecting the lung. In infectious settings in which vascular permeability is increased or where lung capillaries or alveolar epithelial cells are damaged, increased IFITM3 in MKs and platelets may thus serve to restrict influenza infection within the lungs. Interestingly, the transcriptome of platelets from dengue-infected patients compared with patients with influenza showed limited overlap between differentially expressed genes. Although IFITM3 was shared between both groups, these data suggest the concept that transcriptional changes are unique to specific pathogens. Future studies are necessary to determine if specific diseases induce pathogen-specific differential gene expression in platelets and what the resulting functional consequences are.
Previous studies have shown that dengue can bind to and infect platelets through a DC-SIGN–dependent mechanism as well as through glycosaminoglycans such as heparan sulfate.34,53 Influenza, conversely, binds sialic acids on target cells through hemagglutinin. Upon binding to the target cell, both viruses enter the cell through clathrin-mediated endocytosis and are trafficked to the endosome. The endosome has a low pH of ∼5 to 6, which triggers the fusion of the viral and endosomal membranes. This action allows for structural rearrangement of the viral proteins and release of the viral RNA into the cell where it is translated into new viral particles. IFITM3 blocks viral release into the cell by preventing fusion of the viral particles to the endosome. Although this mechanism has been reported in other cell types, further studies are necessary to elucidate if a similar mechanism occurs in MKs and platelets.
Recent studies have suggested that MKs and platelets engage in immune-like activities through presentation of antigens to CD8+ T cells as well as secretion of IL-1 to activate fibroblasts in the development of arthritis.40,63 Our data build upon and extend reports of noncanonical activities of platelets and MKs, showing for the first time that MKs actively sense and respond to invading viral pathogens to augment host defense mechanisms. In addition, MKs secrete IFNα and IFNβ (a type I IFN response) upon sensing viral infection, helping protect bystander stem cells from infection. We further hypothesize that IFN secretion by MKs may play additional roles in viral clearance through release of IFNβ and prevention of early viral dissemination through secretion of IFNα.64,65
In conclusion, viral infections upregulated IFITM3 in human platelets and MKs in the present study. Immunizing healthy subjects against dengue also upregulated IFITM3 in platelets, the first evidence that vaccination alters the human platelet transcriptome. Our observations further establish that human MKs sense and respond to dengue, a viral pathogen that infects cells within the bone marrow niche. Through regulated induction of type I IFNs and IFITM3, MKs participate directly in antiviral immune responses. In doing so, MKs can effectively engage or possibly initiate an immune response that not only prevents virus infection in naive MKs but also limits infection in bystander HSCs. These findings identify new roles for MKs that may be targeted to augment host defense mechanisms during infectious challenge. Because the frequency of the loss-of-function variant in IFITM3 is highest in regions where dengue is endemic, these discoveries may also help inform new investigations underpinning genetic susceptibility to dengue infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Diana Lim for her creativity and excellent figure preparation and Toni Blair for her editorial assistance. They thank all of the dedicated dengue vaccine trial volunteers, Patti Lutton and the other clinical research coordinators, and the staff at the University of Vermont Medical Center Clinical Research Center.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL112311 and HL126547 [M.T.R.]; R37HL044525 [G.A.Z.]), the National Institute on Aging (AG048022) (M.T.R.), the National Institute of Neurological Disorders and Stroke (U10NS086606) (R.A.C.), and the National Institute on Minority Health and Health Disparities (2U54M D007587-03) (R.H.-M.). This work was also supported in part by Merit Review Award Number I01 CX001696 from the United States Department of Veterans Affairs Clinical Sciences R&D Service. The dengue vaccine trial was supported the National Institutes of Allergy and Infectious Diseases Intramural Research Program of the National Institutes of Health (HHSN272200900010C). This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. G.A.Z. was supported by a special visiting professorship in the Science Without Borders Program of the Conselho de Desenvolvimento Cientifico e Tecnológico of the government of Brazil while some of this work was conducted. Research reported in this publication was supported by the National Center for Research Resources of the National Institutes of Health under award number 1S10RR026802-01. The project described was supported by award number P30CA042014 from the National Cancer Institute. The work was also supported by the Flow Cytometry Core at the University of Utah. Oligonucleotides were synthesized by the DNA/Peptide Facility, part of the Health Sciences Center Cores at the University of Utah.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, the US Department of Veterans Affairs, or the United States Government. The sponsor had no role in the design or preparation of the manuscript.
Authorship
Contribution: R.A.C., H.S., E.D.H., J.W.R., B.K.M., A.V.W., R.H.-M., N.D.T., M.C., A.S.E., E.M., S.B., C.H.V., B.D.K., K.K.P., S.S.W., S.A.D., P.F.B., G.A.Z., Y.K., P.T.B., F.A.B., A.S.W., and M.T.R. designed and performed experiments; R.A.C., J.W.R., A.S.W., and M.T.R. analyzed results and created the figures; R.A.C., S.A.D., and M.T.R. wrote the paper; and all authors reviewed and critically edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew T. Rondina, University of Utah Health Sciences Center, Eccles Institute of Human Genetics, 15 North 2030 East, Building 533, Suite 4220, Salt Lake City, UT 84112; e-mail: matthew.rondina@hsc.utah.edu.

![Figure 3. Administration of a live dengue vaccine to healthy subjects upregulates platelet IFITM3. (A) Schematic of the dengue vaccination clinical trial. After providing informed consent, healthy subjects were randomized to receive either a live, attenuated dengue vaccine (TV003, n = 17) or placebo injection (Placebo, n = 8) in a 2:1 randomization scheme. Investigators were blinded to study group assignment. Immediately before injection (day 0 [D0]) and again 14 days after injection (D14), whole blood samples were drawn from subjects. Purified platelets were isolated as described in “Methods.” (B) IFITM3 expression was measured by using quantitative RT-PCR in isolated platelets. The graph shows the change in IFITM3 expression on D14, compared with D0 levels. (C) Schematic of the influenza vaccination study. Whole blood samples were drawn from healthy subjects (n = 8) immediately before (D0) receiving the 2012 to 2013 inactivated influenza vaccine and again 14 days (D14) after vaccination. Purified platelets were isolated as described in “Methods.” (D) IFITM3 expression was measured by using quantitative RT-PCR in isolated platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/19/10.1182_blood-2018-09-873984/3/m_blood873984f3.png?Expires=1767710427&Signature=Y7UwpyNp1MG-hyiYpL2fVppX5ORDe8men2gFOs4v-sXNf4ZEkD49~5b-JCj3BnF1-r-pqXN3pkPaWoH~3wrwwap6G~r2AHd72-zxUMMgGfY2vQNrfkUQloa2MMO5qbFsFCLLRXT1o0eXGzNRFfYTZwMpYnToinsNhnwFu9MlQoa~QDg2XLGzeoiqtNJPC~puH0OqRWU9CoSVA8MwLHGtIH4VM5Mu1xJJyPaF4Yc7gdx-JMROH0ZCrgGilJO~75vNHN0j5aDBTACMHYkVSANmigtS7tI~uouOYcAG5lr2O3-LX9WyBNXle1PTilLhMfikI4qkYjX3U5deg7pYWr12jw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. IFITM3 in MKs functions to limit dengue infection. MEG-01s were left alone (No pZIP), or transfected with an empty vector control (pZIP + EV) or an engineered vector containing IFITM3 (pZIP-IFITM3 [eg, IFITM3 overexpression]). IFITM3 mRNA (A) and IFITM3 protein (B) were determined by using quantitative RT-PCR (qRT-PCR) and immunoblot, respectively, to confirm IFITM3 overexpression in unstimulated MEG-01s after transfection. (C) pZIP-EV control or pZIP-IFITM3 overexpressing MEG-01s were infected with DENV2 (t = 96 hours; MOI 0.1) in the presence of subneutralizing concentrations of monoclonal antibodies against DENV2. Antiflavivirus antibody 4G2 (200 ng) was mixed with DENV2 (MOI 0.1) and incubated with MEG-01s. DENV2 mRNA was measured by using qRT-PCR. (D-E) MEG-01s were transfected with control shRNA (sh-NC) or an engineered vector containing shRNA against IFITM3 (sh-IFITM3). sh-NC and sh-IFITM3 MEG-01s were treated with IFNα or vehicle control, and IFITM3 protein was measured (n = 4). (F) sh-NC or sh-IFITM3 MEG-01s were infected with DENV2 (t = 96 hours; MOI 0.1) via antibody-dependent enhancement mode of infection with DENV2 as described earlier. DENV2 mRNA was measured by using qRT-PCR (n = 6). (G-H) A naturally occurring IFITM3 loss-of-function homozygous variant in human MKs increased susceptibility to dengue infection. (G) Representative Sanger sequencing of CD34+-derived human MKs harboring either the ancestral IFITM3 allele T (WT, left) or the IFITM3 loss-of-function single-nucleotide polymorphism variant rs12252T-C (IFITM3 variant, right). Red rectangles highlight the single nucleotide change in IFITM3 from a thymine (T, ancestral) to a cytosine (C, variant). (H) MKs harboring either ancestral IFITM3 or the loss-of-function IFITM3 variant (n = 4 different subjects per group) were infected with DENV2 (t = 96 hours; MOI 0.1). DENV2 mRNA was measured by using qRT-PCR and normalized to GAPDH.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/19/10.1182_blood-2018-09-873984/3/m_blood873984f7.png?Expires=1767710427&Signature=TDtJfaOXJUV91S5TD5T-UBbLn6ULlBZAJUAZp5Loi67NYF7f~1lc1HS0xKRhyvRHcrWUCijZUzE7m45nLO6MDVjb7-rZAZJJmG5nXMfIJGCvTHkPo4PnvSCjyBvmCpyEENTOUqkBUAWdoRWeRQHA5ZtzMmYWs7W1klR4Q116Z1k~OscM3IXkaxRGO3DNvHd4SxqzZJe8DFWrqflUEKZFZI-mDKCP21UH0wrtNv2JgBBbeNJGI5rMQzhhd75giaB8ETg2Es-EPDOzPXk82BtANEPJlm7~ZTs2T7ZDFop-KD6NUs-AwokoflcmR1vRWMmLUeIVnmDq7UXzvI-ts35lRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal