Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant plasma cell dyscrasia that consistently precedes multiple myeloma (MM) with a 1% risk of progression per year. Recent advances have improved understanding of the complex genetic and immunologic factors that permit progression from the aberrant plasma cell clone to MGUS and overt MM. Additional evidence supports bidirectional interaction of MGUS cells with surrounding cells in the bone marrow niche that regulates malignant transformation. However, there are no robust prognostic biomarkers. Herein we review the current body of literature on the biology of MGUS and provide a rationale for the improved identification of high-risk MGUS patients who may be appropriate for novel clinical interventions to prevent progression or eradicate premalignant clones prior to the development of overt MM.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant clonal disorder.1 It is classified based on the involved immunoglobulin (M-protein): non–immunoglobulin M (IgM), IgM, and light chain, which could progress to multiple myeloma (MM), a lymphoproliferative disorder, amyloidosis, or light-chain deposition disease at a rate of 1% per year.2,3 Patients with IgM MGUS, in particular, have an increased risk of developing non-Hodgkin lymphoma, chronic lymphocytic leukemia, light-chain amyloidosis, and Waldenström macroglobulinemia (WM) at a rate of 1.5% per year.4
The most common heavy-chain subtype in MGUS is IgG found in ∼70% of patients, followed by IgM (15%), IgA (12%), and biclonal gammopathy (3%).3 Non-IgM MGUS is defined as non-IgM serum monoclonal protein <3 g/dL, <10% monoclonal plasma cells (PCs) in the bone marrow (BM) and absence of myeloma-defining events (MDEs) or CRAB criteria: hypercalcemia, renal insufficiency, anemia, and lytic bone lesions.5,6 IgM MGUS describes the presence of IgM monoclonal protein <3 g/dL in the absence of CRAB criteria, myeloma-defining events, constitutional symptoms, hyperviscosity, lymphadenopathy, hepatosplenomegaly, or other end-organ damage attributable to abnormal paraprotein. Light-chain MGUS is defined as abnormal free light chain (FLC) ratio (<0.26 or >1.65) in the presence of an increased serum value of the involved light chain, <10% clonal BM PCs, and the absence of monoclonal immunoglobulin heavy chain in serum and urine, CRAB criteria, and amyloidosis.7 Smoldering multiple myeloma (SMM), an intermediate stage, has 10% to 59% BM involvement and/or an M-protein >3 g/dL and a higher progression rate to MM.
MGUS is both biologically and clinically heterogenous with a spectrum of patients who eventually progress and others who have a durably indolent course. Over the past decade, there has been increasing interest in understanding the initiating biologic events of MGUS as well as the sequential cellular, genomic, and immunologic changes associated with progression.8 Considering these new developments, we offer a comprehensive review of the current biology and guidelines for clinical management of MGUS.
Epidemiology
MGUS is usually diagnosed incidentally when serum protein electrophoresis (SPEP) and immunofixation are sent to work up high serum protein levels or other clinical disorders. The first study on MGUS prevalence originated from Sweden over 50 years ago and included 294 healthy subjects ≥70 years of age9 followed by a study on 6995 adults over the age of 25 years,10 which found an M-protein in 3.1% and 2.5% of subjects, respectively. This was followed by a study in the United States evaluating 21 463 individuals ≥50 years of age in which MGUS was present in 3.2% of individuals, 5.3% of persons ≥70 years, and 8.9% of men ≥85 years old.11,12 Similar prevalence has been reported in Italian13 and French14 cohorts.
MGUS is detected twice as frequently in men compared with women and 3 times more often in patients of African descent.15-18 Interestingly, MGUS in black patients is associated with lower M-protein levels, higher rate of abnormal FLC ratio, younger mean age distribution, and lower IgM gammopathy prevalence.15,18-21 Although the prevalence of MGUS is higher in black patients, the rate of progression to MM is the same.17,22,23 These studies suggest that race and genetic ancestry are important considerations in prognosis, counseling, clinical management, and screening efforts.
Risk factors
A study of over 4 million male US veterans identified 6687 people with a PC disorder and found that MGUS/MM are associated with broad categories of inflammatory, infectious, and autoimmune disorders with median relative risks of 1.18, 1.29, and 1.15, respectively.24 The clinical significance of these associations remains to be validated. A Swedish study found similar associations.25 Notably, among HIV+ individuals, reports of MGUS incidence ranged from 2.5% to 61% in 1 report, with a 4.5-fold increased risk of developing MM.26-28
One retrospective study on veterans found that MM is associated with higher body mass index.29 Another retrospective population-based study showed an independent association of MGUS and obesity irrespective of socioeconomic status.30 In contrast, a retrospective study on 575 Swedish MGUS patients did not find higher rates of MGUS in obese patients but observed an increased progression rate to MM (hazard ratio = 2.66).31 A retrospective study of US veterans similarly found that obesity was associated with increased progression.32 In the same cohort, metformin use for at least 4 years among patients with diabetes with MGUS was associated with a lower risk of progression to MM (hazard ratio = 0.47).33 These studies implicate dysregulated metabolism in the development and progression of MGUS and suggest that weight modification and glycemic control may be potential targets for modifying the disease’s natural history.
Environmental exposures
An association between MGUS and radiation exposure was established in a study on 52 525 survivors of the Nagasaki atomic bomb.34 Although MGUS prevalence in Japan is 2.4% in those >50 years of age,35 there was a higher prevalence of MGUS in people living within 1.5 km of the bomb hypocenter, compared with those living >3 km away, with a mean prevalence ratio of 1.4. This finding was noted in those who were exposed to a high radiation dose (>0.1 Gy) at an age of ≤20 years. However, this study reported a low rate of progression to MM of 0.7% per year that was independent of radiation exposure.
A prospective study on 555 men working with pesticides (dieldrin, carbon-tetrachloride/carbon disulfide, and chlorothalonil) found a 6.8% MGUS prevalence.36 Similarly, a study of 479 veterans exposed to the herbicide Agent Orange found an increased risk (odds ratio = 2.37) and prevalence (7.1%) of MGUS.37
In a 10-year follow-up of 3949 German participants, long-term residential exposure to particulate matter was associated with increased MGUS incidence, and risk increased with larger particle size.38 A recent study on 781 firefighters exposed to airborne carcinogens during the 9/11 World Trade Center attacks found a 7.63% prevalence.39 More specifically, light-chain MGUS prevalence was more than threefold higher. Air contaminants act as carcinogens and can trigger chronic inflammation, autoimmunity, and, potentially, inflammation-induced oncogenesis, emphasizing a possible immune component in myelomagenesis.40-42
Familial MGUS and the mode of inheritance
Familial clustering of MM patients has been reported43 and analyses of the largest familial study from the Swedish database reported a 1.7-fold increase in risk of developing MM among first-degree relatives of MM patients.44 That same database, encompassing 4458 MGUS patients and 14 621 first-degree relatives, later revealed that relatives of MGUS patients had a fourfold and 2.9-fold elevated risk for developing MGUS and MM, respectively.45
A series of investigations aimed at uncovering the mode of inheritance of MGUS/MM discovered that hyperphosphorylated Paratarg-7 (pP-7), a protein of unknown function, is linked to both familial and nonfamilial MGUS and MM.46,47 Analyses of 8 families revealed that pP-7 is inherited in a dominant fashion and leads to the development MGUS and MM (odds ratio = 7.9). Investigators hypothesized that hyperphosphorylation could induce autoimmunity via chronic antigenic stimulation, which in turn may lead to the development a PC disorder. Interestingly, 1 study detected a hyperresponsive B-cell phenotype that was shared by several individuals within families with cases of MGUS/MM.48 Furthermore, pP-7 was detected in 37% of African Americans, compared with 16.7% in Europeans and 4% in Japanese MGUS/MM patients, suggesting a role for this genetic factor among African Americans.49
Genome-wide association studies (GWASs) were also done to analyze single-nucleotide polymorphisms. Numerous loci were found to influence the risk of developing MGUS and MM, including 3p22.1 (rs1052501), 6p21.33 (rs2285803), 7p15.3 (rs4487645), and 17p11.2 (rs4273077).50-52 Interestingly, loci 7p15.3 includes CDCA7L, a MYC-interacting gene, making it a potential region for further analysis due to the major role of MYC in driving myeloma.53,54 Another genome-wide association study identified 23 novel loci interactions regulating B-cell receptor, epidermal growth receptor, and cell adhesion–related pathways, which could be related to MGUS development and progression.55
Genomic landscape
Microarrays and next-generation sequencing
Copy-number abnormalities (CNAs), including gains of 1q, 3p, 6p, 9p, 11q, 19p, 19q, and 21q and deletions of 1p, 16q and 22q, can be detected in MGUS, but at a lower frequency (60.6%) compared with MM (100%).56-58 On the other hand, the most common CNAs for IgM MGUS/WM are del6q, +18q, trisomy 4, 5, 12, and monosomy 8.59,60 However, there seems to be a temporal acquisition of CNAs, some of which are more prevalent at later stages. For example, although del6q is detected in smoldering and symptomatic WM, it is not seen in IgM MGUS, indicating that it may be a secondary event.60,61 Furthermore, although t(11;14) is uniform across the full non-IgM disease spectrum, t(4;14), t(14;16), and del13q are more common in the SMM and MM stages and may follow a nonrandom natural biological history with 1 chromosomal defect routinely preceding another.62-66 Notably, del13q is dependent on the genetic context, whereby it is rare in MGUS and SMM patients with a t(11;14) and t(6;14) compared with MM, whereas it is equally prevalent in all 3 stages in the presence of t(4;14) and t(14;16).64
MM-associated somatic mutations (KRAS, NRAS, DIS3, HIST1H1E, EGR1, and LTB) were detected in few MGUS cases, suggesting a less complex genomic landscape.56 Yet, this may be explained by the low tumor fraction in MGUS, where single-nucleotide variants are more easily missed compared with CNAs. Moreover, MYC translocations and TP53 deletions and mutations were not detected in MGUS, suggesting that these may be drivers of progression. Also, the median number of CNAs was positively correlated with higher risk groups and acquiring somatic mutations. On the other hand, IgM MGUS/WM have a different set of somatic mutations with the most common being the MYD88 L265P mutation, followed by mutations in CXCR4 and KMT2D, in addition to lower frequency mutations including ARID1A, CD79b, MYDBBP1A, NOTCH2, PRDM1, TP53, TRAF3, and TNFAIP3.67 Notably, it was found that most primary translocations result from an aberrant IgH switch recombination event in pre–germinal center B cells leading to increased oncogene expression.68,69
Gene and microRNA expression profiling
Although DNA studies provided a robust understanding of MM pathogenesis, gene-expression profiling (GEP) studies further characterized disease states. One study reported 52 differentially expressed genes between PCs of patients (MGUS, SMM, and MM) and controls.70 They identified 4 signatures that classified patients into: MM-like MGUS, non–MM-like MGUS, MGUS-like MM, and non–MGUS-like MM, whereby the MM-like MGUS have an increased risk of progression and the MGUS-like MM have a longer survival. A 70-gene signature (GEP-70) in newly diagnosed MM patients was also found to correlate well with survival and myeloma staging.71 A prospective study (SWOG0120) later found that a GEP-70 score more than −0.26 and GEP proliferation index more than −2.73 predicted an increased risk of MGUS/SMM transformation into MM.72 One of the most important contributions of GEP was identifying the dysregulation of CCND1 (11q13), CCND3 (6p21), or CCND2 (MAF [16q23] and MAFB [20q11]) as a unifying early event in nonhyperdiploid MGUS.73,74
Expression profiling of 345 microRNAs (miRNAs) in PCs found 41 upregulated and 7 downregulated miRNAs in MGUS compared with normal PCs,75 some of which play a role in B- and T-cell differentiation.76 In particular, miRNA-21, -181a, and -106b∼25 may involve alterations in the p53 pathway, as they are known to target the p300-CBP–associated factor that acetylates p53.75 Moreover, circulating serum miRNA-744, miRNA-130a, miRNA-34a, let-7d, and let-7e were found to be dysregulated in both MGUS and MM, and miRNA-34a and let-7e can particularly distinguish MGUS patients from healthy individuals with a sensitivity of 91.1% and specificity of 96.7%.77 Additional epigenetic studies found specific genes to be hypermethylated in both MGUS and MM, including p15, p16, p53, DAPK, ARF, SOCS-1, E-cadherin, and hMLH-1,78-81 revealing that early MM stages exhibit a similar pattern of tumor-suppressor gene methylation but with a lower methylation index in MGUS.
Tumor microenvironment
The BM is a collection of cellular (immune, endothelial, adipocytes, mesenchymal stem cells, reticular, and osteolineage cells) and noncellular components, extracellular matrix (ECM), and soluble factors, all of which maintain homeostatic hematopoiesis. Therefore, studies have begun focusing on the BM composition as a permissive microenvironment for clonal selection and progression from MGUS to MM.
Osteolineage cells
Osteolytic lesions are 1 of the hallmarks of MM and are mainly driven by receptor activator of NF-κΒ ligand (RANK-L) upregulation and osteoprotegerin (OPG) downregulation in osteoblasts, which activates osteoclasts.82 Although bone lesions are not observed in MGUS, the RANK-L/OPG and bone fracture risk is already higher.83,84 MM mouse model studies have shown that MM cells can secrete Dickkopf-related protein 1 (DKK1), a Wnt/β-catenin pathway inhibitor, and the antiosteoblastic factors transforming growth factor β and hepatocyte growth factor, which in turn can suppress BMP2 and RUNX2 that induce apoptosis and suppress proliferation and differentiation of osteoprogenitors.85-87 Although these activated pathways are mainly studied in mice experiments, it is still imperative that we elucidate their presence in humans.
Stromal, endothelial, and mesenchymal stem and progenitor cells
Mesenchymal stem and progenitor cells comprise a major portion of the BM niche and are responsible for regulating adhesion and migration, via VCAM1 and ICAM1, and survival and proliferation of MM cells through direct cell-cell interactions and secreting growth or antiapoptotic factors (insulin-like growth factor 1, interleukin 6 [IL-6], and CXCL12).88,89 Interestingly, in vitro studies using human cells revealed that these cells are persistently abnormal even in the absence of MM cells, which may explain the nonhealing bone lesions that remain after successfully eradicating the malignant cells.90
GEP studies of human stroma, ranging from MGUS and up to relapsed/refractory MM, did show a differentially expressed signature compared with healthy individuals and these included IL-6, DKK1, HOXB91 and wound healing, tumor necrosis factor α, and hypoxia pathways.92 The BM is a hypoxic environment and becomes even more hypoxic in the presence of MM cells, thereby inducing endothelial cell neoangiogenesis via expression of IL-17, syndecan 1, hypoxia-inducible factor 1, and vascular endothelial growth factor.93,94 This hypoxia is known to drive epithelial to mesenchymal transition of MM cells, decreasing E-cadherin and increasing CXCR4 expression, which promotes myeloma dissemination.95 Although most of these studies investigated the makeup of myeloma BMs, these changes could have already taken place in the MGUS stage, as other studies have found. For example, fibroblasts were found to begin modifying the BM niche in MGUS, reflected by the gradual increase in the ECM-remodeling proteomic makeup.96 Characterizing the proteomic signature of the BM ECM identified a total of 11 proteins in MGUS, compared with healthy controls and MM, including 2 core (proteoglycan 2 and 3) and 9 matrisome-associated proteins such as ficolin 1, cathepsin G, serpins, HRNR, S100A8, and S100A9.97
Immune composition
Evading and suppressing the host immune system is an important step in the progression of MGUS to MM. Usually, natural killer (NK) cells and cytotoxic T lymphocytes are responsible for eliciting an immune response against cancerous cells, however, tumor cells can suppress these anticancer responses. Immune suppression includes loss of antigen presentation, defective immune cell function, depleted myeloma-specific T cells, and increasing immunosuppressive cell types, such as myeloid-derived suppressor cells and regulatory T cells (Tregs).98 T-cell expansion is observed in both MGUS and MM patients, yet it is more robust when tumor burden is low in MGUS and decreases during progression to MM.99-101 Interestingly, although NK-cell expansion is easily discernible in the peripheral blood and BM of MM patients, their activity is decreased due to a tumor cell–mediated downregulation of NKG2D on NK cells.102
Increased immune suppression parallels an increase in T-helper 17 (Th17) cell abundance,103 secretion of several cytokines and growth factors,104 and MM cell induction of Treg expansion via a contact-dependent manner, via inducible costimulator/inducible costimulator ligand.105 Additionally, stromal cells were shown to inhibit both T- and B-lymphocyte function by activating Tregs, leading to a poorer outcome.106 We detected Treg expansion due to MM cell secretion of type 1 interferons in the early disease stages of a murine MM transplantable model and demonstrated that survival of mice injected with Vk*Myc cells was prolonged when the Treg population was depleted.107 Another study found that stromal cells induce the expression of programmed cell death (PD) ligand-1 (PD-L1) on MM cells,108,109 and CD8+ T and NK cells from MM patients demonstrate high levels of PD-1 that contribute to immune tolerance.110 Although these studies have investigated the immune composition in the presence of myeloma cells, recent unpublished studies using novel single-cell RNA-sequencing techniques are beginning to detect the same immune alterations already taking place in the MGUS stage. Importantly, the essential role of the immune microenvironment in driving progression was mimicked in genetically humanized MIS(KI)TRG6 mice that were transplanted with CD3-depleted mononuclear cells and injected with primary human premalignant and malignant PCs.111 The investigators found that injected primary tumor cells of MGUS patients continued to grow progressively.
Presentation and clinical consequences of MGUS
MGUS is associated with infections,112 fractures,113 peripheral neuropathy (PN),114 thromboembolism,115 and monoclonal gammopathy of renal significance (MGRS).116 Retrospective studies linking adverse clinical events to MGUS are challenged by lack of clinical testing to prove causal relationship (except for MGRS) and the potential for overestimation of risk given that patients with infection, thromboembolism, or fracture are more likely to seek medical care. Still, there is some rational basis for risk of adverse events in MGUS. Deficient humoral immune responses in MGUS may lead to high rates of infection in this population and MGUS patients have been shown to have reduced antibody response to vaccination.117 Increased hip and spinal fractures are thought to result from altered bone strength and microarchitecture113 and may be related to elevated RANK-L/OPG ratios.83 MGUS patients have decreased bone mineral density and increased rates of osteoporosis,118 but with increased bone size and cortical porosity and decreased cortical thickness, suggesting that bone density and strength is altered in a manner distinct from decreased mineralization.119
PN is found in 10% of MGUS patients, and more commonly in the IgM type.114 IgM MGUS is associated with distal acquired demyelinating symmetric neuropathy that presents with sensory ataxia and mild distal motor deficits. Anti–myelin-associated glycoprotein (MAG) antibodies are detected in 50% of these patients,120 whereas other PNs may be associated with anti-ganglioside antibodies. There is no proven causal relationship for neuropathy in non-IgM MGUS, so care should be taken to exclude all other potential causes of neuropathy. Rarely, IgG/A MGUS is associated with neuropathy manifesting as a chronic inflammatory demyelinating polyradiculopathy with proximal and distal motor deficits114 or axonal neuropathy involving distal extremities that begins with sensory ataxia and progresses slowly to motor weakness.121
MGRS describes a group of kidney disorders (renal impairment and/or proteinuria) caused by the physiochemical and immunologic properties of deposited monoclonal immunoglobulins in premalignant PC dyscrasias and are diagnosed by renal biopsy.122 MGRS includes glomerulopathies with immunoglobulin depositions such as those with fibrillar amyloidosis (immunoglobulin light chain [AL], heavy chain [AH], and light and heavy chain [ALH]), microtubular (type I and type II cryoglobulinemias, immunotactoid glomerulopathy), or nonorganized deposits (monoclonal immunoglobulin deposition disease [MIDD]). Proliferative glomerulonephritis with monoclonal immunoglobulin deposits and tubular disorders such as Fanconi syndrome are also types of MGRS.116
In a retrospective study of 37 MGRS patients, 22% progressed to end-stage renal disease (ESRD) over an average follow-up of 30.3 months.123 In another retrospective evaluation of 19 MIDD patients, 5-year ESRD-free survival was only 37%.124 Notably, for patients who develop ESRD, MGRS often recurs after renal transplant and is associated with allograft loss.124,125 Importantly, complete hematologic response prior to renal transplant appears to reduce the risk of recurrence and is therefore important for allograft survival.126 Thus, it is important to monitor renal function of MGUS patients and maintain a high index of suspicion for MGRS in patients with otherwise unexplained renal dysfunction.
In the Mayo Clinic risk-stratification model, progression risk is increased with serum monoclonal protein (>1.5 g/dL), non-IgG disease, and abnormal serum FLC ratios (<0.26 or >1.65).23,127 At time of presentation, MGUS patients should be stratified based on number of risk factors: high-risk patients possess all 3 risk factors and have a 20-year progression risk of 58%; high-intermediate-risk have 2 risk factors and a 37% progression risk; low-intermediate-risk have 1 risk factor and a 21% chance of progression; low-risk have no risk factors and a 5% progression risk.23,127 Alternatively, the PETHEMA Study Group risk-stratified patients using a ratio of abnormal/normal PCs >95% and DNA aneuploidy (hyperdiploidy or hypodiploidy). At 5 years, patients with abnormal PCs >95% and aneuploidy were found to have a 46% risk of progression compared with a 10% risk for patients with 1 risk factor and 2% for patients with none.128
Screening for MGUS
Currently, there are no guidelines for screening asymptomatic individuals for MGUS. Yet, there is significant interest in understanding the epidemiologic, genetic, and immunologic factors associated with increased risk of progression, to direct future screening and early intervention efforts in high-risk patients. Two large-scale screening efforts are under way in this regard: Iceland Screens Treats and Prevents Multiple Myeloma (iSTOPMM) and Predicting Progression of Developing Myeloma in a High-Risk Screened Population (PROMISE). The iSTOPMM is screening individuals ≥45 years of age for MGUS and randomizing patients into intensive or standard follow-up and will evaluate the impact of each follow-up practice on overall survival.129 The PROMISE study (www.promisestudy.org) in the United States aims to establish a prospective cohort of patients with MGUS and SMM in high-risk individuals (African-Americans and first-degree relatives of patients with PC disorders) to elucidate clinical, genomic, epigenetic, and immune predictors of progression to MM130 (Table 1). However, outside of a clinical trial, we do not recommend screening for MM in family members of individuals with MGUS/SMM/MM.
Active trials in MGUS
| Title . | Intervention . | Status . | URL . |
|---|---|---|---|
| Registry and prospective studies | |||
| Predicting progression of developing myeloma in a high-risk screened population (PROMISE) | Recruiting | https://clinicaltrials.gov/show/NCT03689595 | |
| Collection of specimens and clinical data to create a bio-repository for multiple myeloma | Recruiting | https://clinicaltrials.gov/show/NCT03616483 | |
| Iceland screens, treats or prevents multiple myeloma | Active, not recruiting | https://clinicaltrials.gov/show/NCT03327597 | |
| Unravel MGUS (monoclonal gammopathy of unknown significance) | Recruiting | https://clinicaltrials.gov/show/NCT02933021 | |
| Prospective observational study of clinical and genomic predictors of progression to myeloma in asymptomatic monoclonal gammopathies | Recruiting | https://clinicaltrials.gov/show/NCT02726750 | |
| Study of MGUS, smoldering myeloma, early MDS and CLL to assess molecular events of progression and clinical outcome | Recruiting | https://clinicaltrials.gov/show/NCT02269592 | |
| A prospective study of circulating multiple myeloma cells as a biomarker of progression in myeloma precursor states (MGUS and SMM) | Active, not recruiting | https://clinicaltrials.gov/show/NCT01958528 | |
| A diagnostic screening trial seeking amyloidosis very early (for patients with LC MGUS and SMM) | Recruiting | https://clinicaltrials.gov/ct2/show/NCT02741999 | |
| Studies with interventions to prevent progression of MGUS to MM | |||
| Phase II study of the CD38 antibody daratumumab in patients with high-risk MGUS and low-risk smoldering multiple myeloma | Daratumumab | Recruiting | https://clinicaltrials.gov/show/NCT03236428 |
| Dendritic cell DKK1 vaccine for monoclonal gammopathy and stable or smoldering myeloma | DKK1 vaccine | Not yet recruiting | https://clinicaltrials.gov/show/NCT03591614 |
| Antigen-lipid-driven monoclonal gammopathies targeting epicardial fat | Liraglutide | Not yet recruiting | https://clinicaltrials.gov/show/NCT02920190 |
| Green tea extract in treating patients with monoclonal gammopathy of undetermined significance and/or smoldering multiple myeloma | Green tea extract | Terminated | https://clinicaltrials.gov/show/NCT00942422 |
| Rituximab in treating patients with peripheral neuropathy caused by monoclonal gammopathy of undetermined significance | Rituximab | Terminated | https://clinicaltrials.gov/show/NCT00588822 |
| RIMAG study: trial of rituximab versus placebo in polyneuropathy associated with anti-MAG IgM monoclonal gammopathy | Rituximab | Completed | https://clinicaltrials.gov/show/NCT00259974 |
| Celecoxib in preventing multiple myeloma in patients with monoclonal gammopathy or smoldering myeloma | Celecoxib | Completed | https://clinicaltrials.gov/show/NCT00099047 |
| Title . | Intervention . | Status . | URL . |
|---|---|---|---|
| Registry and prospective studies | |||
| Predicting progression of developing myeloma in a high-risk screened population (PROMISE) | Recruiting | https://clinicaltrials.gov/show/NCT03689595 | |
| Collection of specimens and clinical data to create a bio-repository for multiple myeloma | Recruiting | https://clinicaltrials.gov/show/NCT03616483 | |
| Iceland screens, treats or prevents multiple myeloma | Active, not recruiting | https://clinicaltrials.gov/show/NCT03327597 | |
| Unravel MGUS (monoclonal gammopathy of unknown significance) | Recruiting | https://clinicaltrials.gov/show/NCT02933021 | |
| Prospective observational study of clinical and genomic predictors of progression to myeloma in asymptomatic monoclonal gammopathies | Recruiting | https://clinicaltrials.gov/show/NCT02726750 | |
| Study of MGUS, smoldering myeloma, early MDS and CLL to assess molecular events of progression and clinical outcome | Recruiting | https://clinicaltrials.gov/show/NCT02269592 | |
| A prospective study of circulating multiple myeloma cells as a biomarker of progression in myeloma precursor states (MGUS and SMM) | Active, not recruiting | https://clinicaltrials.gov/show/NCT01958528 | |
| A diagnostic screening trial seeking amyloidosis very early (for patients with LC MGUS and SMM) | Recruiting | https://clinicaltrials.gov/ct2/show/NCT02741999 | |
| Studies with interventions to prevent progression of MGUS to MM | |||
| Phase II study of the CD38 antibody daratumumab in patients with high-risk MGUS and low-risk smoldering multiple myeloma | Daratumumab | Recruiting | https://clinicaltrials.gov/show/NCT03236428 |
| Dendritic cell DKK1 vaccine for monoclonal gammopathy and stable or smoldering myeloma | DKK1 vaccine | Not yet recruiting | https://clinicaltrials.gov/show/NCT03591614 |
| Antigen-lipid-driven monoclonal gammopathies targeting epicardial fat | Liraglutide | Not yet recruiting | https://clinicaltrials.gov/show/NCT02920190 |
| Green tea extract in treating patients with monoclonal gammopathy of undetermined significance and/or smoldering multiple myeloma | Green tea extract | Terminated | https://clinicaltrials.gov/show/NCT00942422 |
| Rituximab in treating patients with peripheral neuropathy caused by monoclonal gammopathy of undetermined significance | Rituximab | Terminated | https://clinicaltrials.gov/show/NCT00588822 |
| RIMAG study: trial of rituximab versus placebo in polyneuropathy associated with anti-MAG IgM monoclonal gammopathy | Rituximab | Completed | https://clinicaltrials.gov/show/NCT00259974 |
| Celecoxib in preventing multiple myeloma in patients with monoclonal gammopathy or smoldering myeloma | Celecoxib | Completed | https://clinicaltrials.gov/show/NCT00099047 |
CLL, chronic lymphocytic leukemia; LC-MGUS, light-chain MGUS; MDS, myelodysplastic syndrome; RIMAG, Rituximab vs Placebo in Polyneuropathy Associated With Anti-MAG IgM Monoclonal Gammopathy.
Management of MGUS
The current standard of care for MGUS is monitoring for progression to enable early detection and intervention. However, growing impetus for investigating early intervention strategies is derived from improved overall survival and reduced complications in patients who were monitored prior to the diagnosis of MM.131,132
Because the risk of progression varies per patient, the extent and frequency of evaluation is based on individual risk (Table 2). At the time of diagnosis, all MGUS patients should have a complete blood count, serum creatinine, and calcium. For high- and intermediate-risk patients, including those with IgM MGUS, we add a baseline lactate dehydrogenase, β-2-microglobulin, and BM biopsy with fluorescence in situ hybridization to the initial assessment. Additionally, we recommend a skeletal survey or preferentially a low-dose computed tomography (CT) for non-IgM, high- and intermediate-risk patients. IgM patients have a much lower risk of bone involvement and so, like low-risk patients, skeletal assessment is not required in the absence of bone symptoms. However, IgM MGUS patients should undergo CT of the chest/abdomen to evaluate for lymphadenopathy, which could indicate lymphoma or WM. Lastly, due to the risk of AL amyloidosis in light-chain MGUS, these patients should be evaluated with baseline N-terminal pro–B-type natriuretic peptide (NTproBNP), cardiac troponins, and urine protein electrophoresis.7,133 If additional workup is within normal limits, a second evaluation for progression is still recommended in 6 months for all patients.6 Patients who have progressive rise in M-protein over consecutive measurements have a higher risk of progression than those with stable M-protein.134 Follow-up intervals can then be lengthened toward lifetime annual follow-up for high- and intermediate-risk patients, and every 2 to 3 years, or when symptoms of progression arise, for low-risk patients with stable M-protein, as the risk of progression is highest for the first year after diagnosis and declines thereafter.6,132
Risk-stratified management of MGUS patients
| All MGUS patients . | Risk stratification . | Classification . | Additional evaluation at diagnosis . | Monitoring and evaluation . | |
|---|---|---|---|---|---|
| SPEP, CBC, creatinine | Risk factors for progression3 : | 0 risk factors | Low risk | No additional testing required | Repeat SPEP, CBC, and creatinine in 6 mo and then every 2-3 y if stable, or when symptoms of progression arise |
| • M-protein, >1.5 g/dL | 1 risk factor | Low-intermediate risk | LDH B2-macroglobulin Bone marrow biopsy with FISH IgM MGUS → CT chest and abdomen to evaluate for lymphadenopathy Non-IgM MGUS → skeletal assessment† Light-chain MGUS → NTproBNP, cardiac troponins, urine albumin | If additional testing negative → SPEP, CBC, and creatinine in 6 mo then annually for life if remains stable* If signs of progression → decrease follow-up interval and initiate workup for lymphoplasmacytic malignancy | |
| • Non-IgG paraprotein (IgA or IgM) | 2 risk factors | High-intermediate risk | |||
| • FLC ratio, <0.26 or >1.65 | 3 risk factors | High risk | |||
| All MGUS patients . | Risk stratification . | Classification . | Additional evaluation at diagnosis . | Monitoring and evaluation . | |
|---|---|---|---|---|---|
| SPEP, CBC, creatinine | Risk factors for progression3 : | 0 risk factors | Low risk | No additional testing required | Repeat SPEP, CBC, and creatinine in 6 mo and then every 2-3 y if stable, or when symptoms of progression arise |
| • M-protein, >1.5 g/dL | 1 risk factor | Low-intermediate risk | LDH B2-macroglobulin Bone marrow biopsy with FISH IgM MGUS → CT chest and abdomen to evaluate for lymphadenopathy Non-IgM MGUS → skeletal assessment† Light-chain MGUS → NTproBNP, cardiac troponins, urine albumin | If additional testing negative → SPEP, CBC, and creatinine in 6 mo then annually for life if remains stable* If signs of progression → decrease follow-up interval and initiate workup for lymphoplasmacytic malignancy | |
| • Non-IgG paraprotein (IgA or IgM) | 2 risk factors | High-intermediate risk | |||
| • FLC ratio, <0.26 or >1.65 | 3 risk factors | High risk | |||
CBC, complete blood count; FISH, fluorescence in situ hybridization; LDH, lactate dehydrogenase.
Include NTproBNP, cardiac troponins, and urine albumin for light-chain disease.
Low-dose CT preferred.
MGUS patients with neuropathy should undergo extended evaluation including electromyogram/neuromuscular testing, fat pad biopsy, cryoglobulins, and ganglioside/MAG antibody to rule out amyloidosis, cryoglobulinemia, and neurological disorders.114,135 IV immunoglobulin G (IVIG) and rituximab have been used as first-line management for IgM neuropathy whereas plasmapheresis, IVIG, and steroids have been used in IgG/A neuropathy.114,121 However, these therapies do not eradicate the paraprotein-producing clone. There are case reports on the use of high-dose chemotherapy alone136 or followed by autologous stem cell transplant137 for severe debilitating neuropathy in MGUS, but data for these strategies are limited (Table 3).
Clinical disorders associated with MGUS
| Clinical disorders . | Treatment . |
|---|---|
| Monoclonal gammopathies of renal significance | Reference116 |
| Immunoglobulin light-chain amylodosis (AL) | Stage 1 and II disease: melphalan + dexamethasone If stage III or severe renal dysfunction: cyclophosphamide/bortezomib/dexamethasone |
| Immunoglobulin heavy-chain amyloidosis (AH) | |
| Immunoglobulin light and heavy chain (ALH) | |
| Type 1 cryoglobulinemia | If plasmacytic IgG or IgA: antimyeloma regimens If lymphoplasmacytic IgM: rituximab containing regimen |
| Type 2 cryoglobulinemia | Rituximab-containing regimen Treat underlying hepatitis C |
| Immunotactoid glomerulonephropathy (ITG) | Cyclophosphamide/bortezomib/dexamethasone |
| Monoclonal immunoglobulin deposition disease (MIDD) | Cyclophosphamide/bortezomib/dexamethasone Successful use of autologous stem cell transplant reported |
| Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) | Cyclophosphamide/bortezomib/dexamethasone |
| Fanconi syndrome (FS) | Cyclophosphamide/bortezomib/dexamethasone |
| Paraproteinemic neuropathy | Reference114 |
| Distal demyelinating symmetric neuropathy with IgM (DADS-M) | IVIG, consider rituximab |
| IgG/A axonal neuropathy | Plasmapheresis, IVIG, steroids |
| IgG/A chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) | |
| Severe and refractory neuropathy | Consider clinical trial or antimyeloma regimens Successful use of autologous stem cell transplant reported |
| Clinical disorders . | Treatment . |
|---|---|
| Monoclonal gammopathies of renal significance | Reference116 |
| Immunoglobulin light-chain amylodosis (AL) | Stage 1 and II disease: melphalan + dexamethasone If stage III or severe renal dysfunction: cyclophosphamide/bortezomib/dexamethasone |
| Immunoglobulin heavy-chain amyloidosis (AH) | |
| Immunoglobulin light and heavy chain (ALH) | |
| Type 1 cryoglobulinemia | If plasmacytic IgG or IgA: antimyeloma regimens If lymphoplasmacytic IgM: rituximab containing regimen |
| Type 2 cryoglobulinemia | Rituximab-containing regimen Treat underlying hepatitis C |
| Immunotactoid glomerulonephropathy (ITG) | Cyclophosphamide/bortezomib/dexamethasone |
| Monoclonal immunoglobulin deposition disease (MIDD) | Cyclophosphamide/bortezomib/dexamethasone Successful use of autologous stem cell transplant reported |
| Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) | Cyclophosphamide/bortezomib/dexamethasone |
| Fanconi syndrome (FS) | Cyclophosphamide/bortezomib/dexamethasone |
| Paraproteinemic neuropathy | Reference114 |
| Distal demyelinating symmetric neuropathy with IgM (DADS-M) | IVIG, consider rituximab |
| IgG/A axonal neuropathy | Plasmapheresis, IVIG, steroids |
| IgG/A chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) | |
| Severe and refractory neuropathy | Consider clinical trial or antimyeloma regimens Successful use of autologous stem cell transplant reported |
MGUS patients who present with renal impairment should be evaluated for AL amyloidosis and MGRS. After confirming MGRS by kidney biopsy, close cooperation with nephrologists is suggested to determine the optimal treatment strategy based on MGRS subtype, degree of renal impairment, and risk of progression to ESRD. Renal impairment is usually irreversible as there is no available therapy for clearing monoclonal deposits. Treatment options usually involve the use of chemotherapies or immunotherapies for targeting the clonal cell population to reduce paraprotein production and preserve renal function.116 In 1 case series involving 4 patients with dialysis-dependent MIDD, high-dose melphalan plus autologous stem cell transplant was found to be a safe and effective option resulting in durable responses and allowing subsequent renal transplantation138 (Table 3).
The lack of treatment approaches that are safe and effective for MGUS-related neuropathy and MGUS with renal impairment highlight an area of need for novel therapeutic agents to be tested specifically in these patients’ subgroups. Currently, daratumumab is being tested in some of these cases but its efficacy is still unknown.
There are no US Food and Drug Administration (FDA)-approved treatments to eradicate MGUS or prevent progression, and patients currently receive therapeutic intervention in the context of a clinical trial only. The disadvantage of clinical trials is that many patients will not progress to overt malignancy without any therapeutic interventions and are being unnecessarily exposed to potentially toxic therapy. Studies to better understand risks of progression in patients with MGUS and specifically define those at risk of developing MM in their lifetime should be performed so that more selective approaches are used for these patients’ populations. To reduce potential for harm, investigators have considered more innocuous therapeutics for prevention of progression including green tea extract, curcumin,139 and nonsteroidal anti-inflammatory drugs.140 Currently, when anti-MM therapies are used in prevention trials, the patients at highest risk of progression are targeted. For example, a study evaluating the use of the CD38 antibody daratumumab in high-risk MGUS and smoldering patients is ongoing (Table 3).141 Even when targeting high-risk patients, the most appropriate trial designs involve minimally toxic interventions, and clinical trial subjects must be fully informed on their individual risk of progression with the risk of therapeutic side effects.
Conclusion
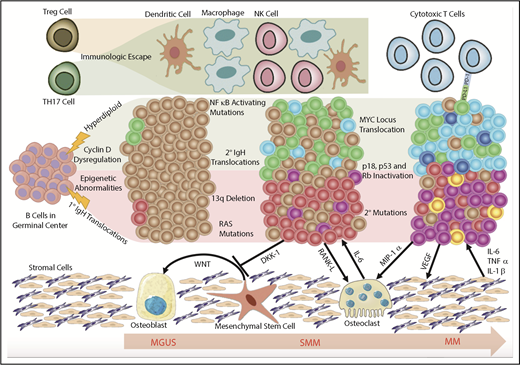
MGUS is an asymptomatic premalignant disorder that increases the risk of developing malignant plasma and B-cell disorders. A multitude of studies have advanced our understanding of the pathophysiology and risk factors for developing MGUS and progression to overt malignancy. A major shift in our understanding of pathogenesis was recognizing clonal heterogeneity and a dynamic equilibrium between tumor cells and the surrounding microenvironment, which enforces evolutionary pressures permitting clonal evolution in a branched heterogeneous process (Figure 1). Improved understanding of the biology of MGUS has allowed clinical investigation into therapeutic approaches to delay progression or even eradicate malignant clones. Such trials have only targeted high-risk patients stratified using the Kyle criteria.127 However, these criteria do not always capture patients who have MGUS-associated morbidity who may also benefit from early intervention. Future studies may seek to identify biomarkers that predict not only risk of progression but also MGUS-associated morbidity such that these patients may be included in clinical trials. Together, these translational efforts will improve our ability to screen and triage MGUS patients and propose clinically meaningful interventions while minimizing toxicity.
Model of clonal evolution. Myelomagenesis is hypothesized to begin with an initiating event in a germinal center B cell that differentiates into a defected PC carrying chromosomal aberrations and gene-expression and epigenetic signatures that separate it from benign PCs. In the progression from MGUS to SMM, the defected PC clone acquires additional chromosomal aberrations and genetic mutations. This is accompanied by a permissive microenvironment in the BM niche that involves bidirectional crosstalk between the malignant clones and surrounding cells that induces immune suppression and clonal expansion to overt MM. MIP-1α, macrophage inflammatory protein 1α; Rb, retinoblastoma protein; TH17, T helper 17; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Model of clonal evolution. Myelomagenesis is hypothesized to begin with an initiating event in a germinal center B cell that differentiates into a defected PC carrying chromosomal aberrations and gene-expression and epigenetic signatures that separate it from benign PCs. In the progression from MGUS to SMM, the defected PC clone acquires additional chromosomal aberrations and genetic mutations. This is accompanied by a permissive microenvironment in the BM niche that involves bidirectional crosstalk between the malignant clones and surrounding cells that induces immune suppression and clonal expansion to overt MM. MIP-1α, macrophage inflammatory protein 1α; Rb, retinoblastoma protein; TH17, T helper 17; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Acknowledgments
The authors acknowledge Romanos Sklavenitis-Pistofidis and Mark W. Bustoros for their contribution in the writing of this manuscript as well as Muhieddine M. Itani who drew the figure for this manuscript.
Authorship
Contribution: T.H.M., L.D.W., and I.M.G. researched data and wrote, reviewed, and/or revised the manuscript before submission.
Conflict-of-interest disclosure: I.M.G. has a consulting/advisory role with Celgene, Takeda, Bristol-Myers Squibb, Janssen Pharmaceuticals, and Amgen, and received research funding/honoraria from Celgene, Takeda, Bristol-Myers Squibb, Janssen Pharmaceuticals, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.
REFERENCES
Author notes
T.H.M. and L.D.W. are joint first authors.