In this issue of Blood, Møller et al demonstrate lower hemoglobin levels, red blood cell (RBC) transfusions, and cerebral tissue oxygenation, as well as potentially higher morbidity, in low vs high transfusion triggers in vascular surgery patients.1
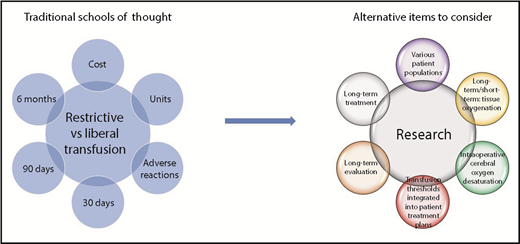
Expanding the picture: most randomized controlled trials regarding restrictive vs liberal transfusion thresholds focus on 30-day mortality. Consideration of different patient populations, incorporation of patient evaluations and treatments, and additional outcomes will likely improve patient outcome.
Expanding the picture: most randomized controlled trials regarding restrictive vs liberal transfusion thresholds focus on 30-day mortality. Consideration of different patient populations, incorporation of patient evaluations and treatments, and additional outcomes will likely improve patient outcome.
In a single center, 58 patients having lower limb bypass or open infrarenal abdominal aortic aneurysm repair were randomized when their hemoglobin was <9.7 g/dL to receive an RBC transfusion at hemoglobin < 8.0 g/dL (low) or <9.7 g/dL (high) trigger. Primary outcome was mean hemoglobin within 15 days of surgery; secondary outcomes were number of RBC units transfused, intraoperative regional or cerebral oxygenation, and severe adverse events within 30 days; and exploratory outcomes, using registry follow-up, were death and major vascular complications (severe transfusion reactions, acute myocardial infarction, stroke, new-onset renal replacement therapy, unscheduled vascular surgery, and amputation) at 90 days. In the low vs high trigger group, mean postoperative hemoglobin was lower by ∼1 g/dL, and a mean of 2 fewer RBC units was transfused per participant (50% less RBC use overall). In addition, the low trigger group had a higher cerebral desaturation load and more major vascular complications at 90 days, but there was no difference between the groups with regard to muscle desaturation load, 30-day adverse events, or mortality; however, the low trigger group lived fewer days outside of the hospital.
Patient blood management entails delivery of the correct product to the correct patient at the correct time.2 This is only doable if we understand the needs of different patient populations. As the field has moved toward restrictive transfusion thresholds for an expanding number of patients, this study creates pause. This study highlights that there may be patient populations who benefit from liberal thresholds and that end points other than mortality should be considered (see figure).
Clinical Practice Guidelines on RBC transfusions recommend restrictive transfusion thresholds. In analyzing randomized controlled trials of transfusion thresholds using the Grading of Recommendations Assessment, Development, and Evaluation method, transfusion is not recommended until the restrictive RBC transfusion threshold of 7 g/dL, vs the liberal threshold of 10 g/dL, is met for hemodynamically stable hospitalized adult patients.3 These recommendations have some exceptions, including patients with acute coronary syndrome, severe thrombocytopenia, or chronic transfusion-dependent anemia. Since the publication of the Transfusion Requirements in Critical Care trial in 1999, >25 studies have shown no differences in 30-day mortality with restrictive vs liberal transfusion thresholds.3
All patient populations may not be the same. Subgroup analysis by clinical setting did not find evidence to support different recommendations in different subpopulations for 30-day mortality.3 However, there were exceptions, such as lower mortality in patients with gastrointestinal bleeding in the restrictive transfusion threshold group.4 A recent systematic review of randomized trials of transfusion thresholds in patients undergoing cardiac surgery or vascular surgery or having myocardial infarction, as well as in other populations, showed no difference in 30-day mortality, with the exception of patients with gastrointestinal bleeding.5 Additionally, there were no differences in other reported outcomes. Thus, restrictive transfusion thresholds permeate transfusion guidelines, although there may be patients who would benefit from liberal thresholds.6
Restrictive transfusion thresholds, as confirmed by the current study, result in fewer patients transfused and less blood use and, thus, reduced costs and fewer transfusion-associated adverse events. However, hemoglobin may not be the best indicator of the need for transfusion. A review of RBC transfusion effects in critically ill patients revealed that patients with abnormal tissue oxygenation prior to transfusion oxygenation improved significantly posttransfusion, suggesting that tissue oxygenation may be a better indicator of transfusion needs than hemoglobin.7 Another review suggests that intraoperative cerebral oxygen desaturation may result in postoperative cognitive dysfunction.8 The primary reason to transfuse is to increase oxygen delivery; the short- and long-term effects of tissue oxygenation remain largely unknown.
Most randomized clinical trials regarding RBC transfusion have looked at 30-day mortality as their clinical outcome. The question remains “Is this the optimal clinical end point?” The current study highlights that other clinically meaningful outcomes may be of use.1 Additionally, individuals with the low hemoglobin trigger had not recovered their baseline hemoglobin at 30 days. A recent retrospective cohort study evaluating postdischarge outcomes at 6 months demonstrated an increase in anemia with decreased RBC transfusion from implemented patient blood management programs. This anemia was not accompanied by an increase in RBC use, rehospitalization, or mortality,9 highlighting the lack of anemia management postdischarge. Anemia has consequences, especially for patients with comorbidities, which may be the cause of the higher 90-day morbidity in this study.10 Therefore, it is important to ask whether morbidity is the appropriate outcome for short-term treatment of transfusion, rather than a more comprehensive evaluation of efficacy, and whether transfusion thresholds should be more integrated into larger patient treatment plans that include hospital and postdischarge care.
In conclusion, Møller et al’s feasibility study creates awareness of transfusion’s role in patient care; how transfusion is integrated into each patient’s care to optimize long-term outcomes remains an area for study. We need to keep learning about different patient population needs, the appropriate indications for transfusion, and how to support these patients in the months following their event.
Conflict-of-interest disclosure: The author declares no competing financial interests.