Circadian behavior in hematopoietic cell function has been described for decades. However, the roles for central vs local nervous have been unclear. In this issue of Blood, García-García et al reveal that the autonomic nervous system works cooperatively with local trafficking responses of hematopoietic stem and progenitor cells (HSPCs) and leukocytes.1
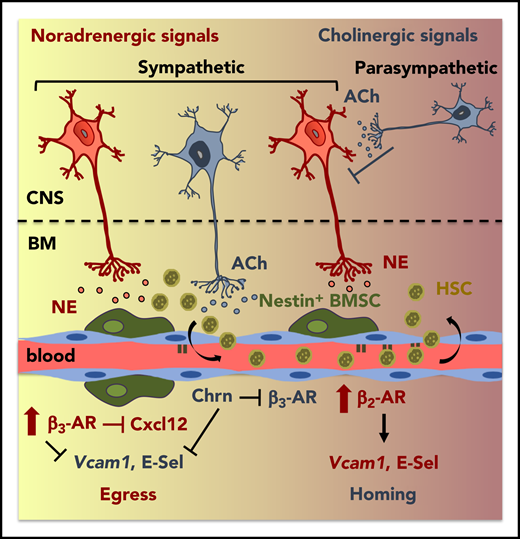
Cholinergic regulation of circadian HSPC traffic. Overview of identified mechanisms, proteins, and cells that influence circadian trafficking (egress and homing) of HSPCs and leukocytes. ACh, acetylcholine; BMSC, BM mesenchymal stem/progenitor cell; Chrn, cholinergic nicotinic; CNS, central nervous system; E-sel, E-selectin; HSC, hematopoietic stem cell; NE, norepinephrine. See the visual abstract for the article by García-García et al that begins on page 224.
Cholinergic regulation of circadian HSPC traffic. Overview of identified mechanisms, proteins, and cells that influence circadian trafficking (egress and homing) of HSPCs and leukocytes. ACh, acetylcholine; BMSC, BM mesenchymal stem/progenitor cell; Chrn, cholinergic nicotinic; CNS, central nervous system; E-sel, E-selectin; HSC, hematopoietic stem cell; NE, norepinephrine. See the visual abstract for the article by García-García et al that begins on page 224.
Multiple types of stem cells, including but not limited to hematopoietic cells, have demonstrated conditional responses and functions including changes in location, maintenance, and regeneration associated with circadian rhythm. Circulation between the blood and bone marrow (BM), differentiation, and fate decisions of HSPCs have been shown to be modified by circadian rhythm as early as the 1960s.2,3 Furthermore, murine HSPCs and leukocytes are typically found in the periphery during the day4 and home to the BM at night5 as a result of sympathetic nervous system involvement. Additional studies have established alterations in engraftment,6 DNA synthesis,7 and other critical cellular functions based on circadian rhythm and sleep deprivation8 in both normal and malignant9 hematopoietic cells. Therefore, it is important to identify and understand the myriad biological mechanisms (both cell autonomous and cell nonautonomous) that regulate stem cells via circadian rhythmicity, in physiological and pathophysiological settings. This understanding would enable the enhancement of therapies to modulate hematopoietic homing/egress and identify how current therapies may have unintended implications for the hematopoietic system.
Previous studies have focused primarily on the role of noradrenergic fibers that innervate the BM and cholinergic muscarinic signals that play a role in the regulation of granulocyte colony-stimulating factor–induced mobilization.10 However, prior to this study, the role of cholinergic signaling in steady state trafficking was unknown. The investigation by García-García et al highlights the role of both branches of the autonomic nervous system in the daily migration of HSPCs and leukocytes (see figure). The authors investigated adrenergic receptor (AR)-mediated interactions during the daily peaks of activity, consistent with norepinephrine (nonadrenergic) and epinephrine (adrenergic) fluctuations and their differential roles in HSPC migration via different (β2 and β3) ARs to elucidate central and local influences on homing and egress.
The GDNF family receptor α 2 (GFRα2) functions in development/survival of cholinergic neurons. The authors used Gfra2−/− mice (with impaired parasympathetic cholinergic neurotransmission) and differing day/night time points to investigate alterations in HSPC/leukocyte egress and homing. During the night, Gfra2−/− mice exhibited diminished urine acetylcholinesterase, suggesting a deficiency in parasympathetic activity. This finding correlated with an increase in cells in the periphery as measured by a colony-forming unit assay. Diminished nocturnal CXCL12 and stem cell factor (Kitl) messenger RNA (mRNA) and protein (CXCL12 only) expression correlated with increased HSPC circulation in the Gfra2−/− mice and these mice exhibited decreased numbers of the nonendosteal Nestin-GFPlo cell population (Lepr+ cells). Interestingly, using a transplantation model of nonirradiated Gfra2−/− mice as recipients, they also observed a doubling of Lin−Sca+Kit+ (LSK) nocturnal homing to the BM that could be normalized (between control and Gfra2−/−) with adhesion-blocking antibodies, illustrating the role for Vcam, and adhesion, in this system. To further investigate the effect of cholinergic neural deficiency on the balance of nocturnal egress vs homing, the authors generated chimeric mice using reciprocal BM transplantation of the Gfra2−/− mice to decipher hematopoietic cell autonomous vs nonautonomous effects. Alterations in nocturnal accumulation of circulating HSPCs were only detected in the Gfra2−/− recipient mice, suggesting that the effect was reliant on non–cell autonomous/microenvironmental signals. Furthermore, day/night oscillations in control vs Gfra2−/− mice showed blunted Adrb2 (β2-AR) mRNA nocturnally in the Gfra2−/− mice and inverted Adrb2 mRNA in Gfra2−/− mice with levels decreased during the day and increased at night (approximately threefold) compared with controls. This finding helps to explain the enhanced egress vs homing in the cholinergic-deficient mice as well as why Gfra2−/−;Adrb2−/− mice had nocturnal circulating HSPCs increased similarly to that of the Gfra2−/− mice as opposed to Gfra2−/−;Adrb3−/− mice, which were similar to control. Observing more local changes, the skulls of Gfra2−/− mice had diminished sympathetic cholinergic fibers. By contrast, neurotrophic factor neuturin−/− (Nrtn−/−) mice, which exhibit increased Gfrα2, had doubled periosteal cholinergic fibers (with normal central sympathetic/parasympathetic activity) relative to control and were used as a gain-of-function model (for Gfrα2). Markedly, the Nrtn−/− mice additionally exhibited normal nocturnal HSPCs in blood, diminished Vcam abundance, and diurnal decreases in HSPC homing, contrary to increased homing in the Gfra2−/− mice, which lack sympathetic cholinergic signaling.
Altogether, this study is the first of its kind to investigate, in-depth, the roles, and balance, of cholinergic regulation with noradrenergic signals in the day/night trafficking of HSPCs and leukocytes (see figure). Furthermore, these data provide a foundation for further studies investigating the mechanisms by which short- vs long-term alterations or disruptions to circadian rhythm and light/dark cycle modify trafficking, signaling, quiescence/exhaustion, and function of normal and malignant stem cells. How these data correlate to human cells, as well as the potential long-term and clinical implications of these responses, will be important avenues for future investigations.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal