Abstract
Venous thromboembolism (VTE), which includes deep vein thrombosis and pulmonary embolism, is a common complication of cancer and is associated with significant morbidity and mortality. Several cancer-related risk factors contribute to the development of VTE including cancer type and stage, chemotherapy, surgery, and patient-related factors such as advanced age and immobilization. Patients with cancer frequently undergo diagnostic imaging scans for cancer staging and treatment response evaluation, which is increasing the underlying risk of VTE detection. The management of cancer-associated VTE is challenging. Over the years, important advances have been made and, recently, randomized controlled trials have been published helping clinicians’ management of this patient population. In this review, we will discuss common cancer-associated VTE scenarios and critically review available evidence to guide treatment decisions.
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication in cancer patients. The risk of developing VTE in these patients is fourfold to sevenfold increased compared with noncancer patients with a reported incidence of up to 15% per year.1,2 Several cancer-associated risk factors for VTE have been identified, including patient-, treatment-, and tumor-related factors (Table 1). At present, cancer patients frequently undergo imaging for tumor staging and evaluation of treatment response, which is further increasing the underlying risk of VTE detection.3 When VTE is diagnosed, anticoagulant therapy is indicated in almost all cases. However, the management of VTE is challenging in this patient population. The risk of recurrent VTE despite anticoagulant therapy and bleeding complications is higher among cancer patients compared with those without cancer.4 In this review, we will discuss 3 common cancer-associated VTE patient scenarios and critically assess the evidence and recent advances to guide treatment decisions (Figure 1).
Risk factor for VTE in cancer patients
| Risk factors . |
|---|
| Patient-related |
| Advanced age |
| Comorbidities |
| Immobilization or hospitalization |
| Previous VTE |
| Hereditary thrombophilia |
| Tumor-related |
| Tumor type |
| • Very high risk: gastric, pancreas, brain |
| • High risk: lung, hematologic, gynecologic, renal, bladder |
| Cancer stage |
| Histological tumor grade |
| Localized tumor compression |
| Treatment-related |
| Chemotherapy (eg cisplatin-based, antiangiogenesis agents) |
| Hormonal therapy |
| Red blood cell transfusions and erythropoiesis-stimulating agents |
| Surgery |
| Radiotherapy |
| Central venous catheters |
| Risk factors . |
|---|
| Patient-related |
| Advanced age |
| Comorbidities |
| Immobilization or hospitalization |
| Previous VTE |
| Hereditary thrombophilia |
| Tumor-related |
| Tumor type |
| • Very high risk: gastric, pancreas, brain |
| • High risk: lung, hematologic, gynecologic, renal, bladder |
| Cancer stage |
| Histological tumor grade |
| Localized tumor compression |
| Treatment-related |
| Chemotherapy (eg cisplatin-based, antiangiogenesis agents) |
| Hormonal therapy |
| Red blood cell transfusions and erythropoiesis-stimulating agents |
| Surgery |
| Radiotherapy |
| Central venous catheters |
Adapted from Ay et al.55 Shown are risk factors for VTE that may be considered in the decision to stop or continue anticoagulation after an initial 3- to 6-month treatment of VTE in cancer patients.
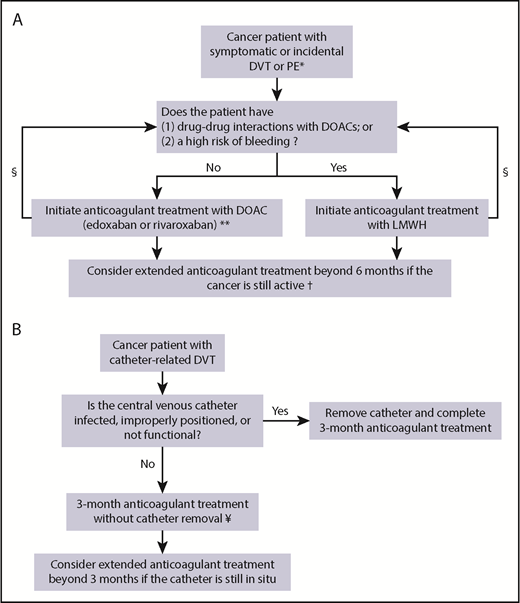
Treatment algorithm. (A) Suggested treatment algorithm for symptomatic and incidental DVT or PE in cancer patients. (B) Suggested treatment algorithm for catheter-related thrombosis in cancer patients. *In patients with isolated single subsegmental PE without concomitant DVT, consider withholding anticoagulant therapy in patients at high risk of bleeding. **Edoxaban is initiated after a LMWH lead-in of at least 5 days. §Assess drug-drug interactions and bleeding risk during follow-up and consider changing the anticoagulant treatment regimen accordingly. †The decision to continue anticoagulant treatment beyond 6 months should also balance the risk of recurrent VTE and bleeding complications in combination with patients’ preference, life expectancy, and treatment costs. ¥LMWH is currently the preferred treatment option. DOAC, direct oral anticoagulant; LMWH, low-molecular-weight heparin.
Treatment algorithm. (A) Suggested treatment algorithm for symptomatic and incidental DVT or PE in cancer patients. (B) Suggested treatment algorithm for catheter-related thrombosis in cancer patients. *In patients with isolated single subsegmental PE without concomitant DVT, consider withholding anticoagulant therapy in patients at high risk of bleeding. **Edoxaban is initiated after a LMWH lead-in of at least 5 days. §Assess drug-drug interactions and bleeding risk during follow-up and consider changing the anticoagulant treatment regimen accordingly. †The decision to continue anticoagulant treatment beyond 6 months should also balance the risk of recurrent VTE and bleeding complications in combination with patients’ preference, life expectancy, and treatment costs. ¥LMWH is currently the preferred treatment option. DOAC, direct oral anticoagulant; LMWH, low-molecular-weight heparin.
Case 1: DVT
A 61-year-old man with a recent diagnosis of stage IIA prostate carcinoma presents to the emergency room with a 3-day history of acute pain, swelling, and erythema of the right lower limb. Compression ultrasonography demonstrates filling defect from the calf trifurcation to the common femoral veins consistent with a diagnosis of a proximal lower-limb DVT. What would be the appropriate anticoagulant treatment regimen for this patient?
Evidence
For many years, low-molecular-weight heparins (LMWHs) have been the first-line treatment of cancer-associated thrombosis.5-9 The LMWHs have been shown to be associated with a lower risk of recurrent VTE (risk ratio [RR], 0.60; 95% confidence interval [CI], 0.45-0.79) without an associated increased risk of major bleeding complications (RR, 1.07; 95% CI, 0.66-1.73) when compared with vitamin K antagonists (VKAs).10 Although direct oral anticoagulants (DOACs) have been well established as first-choice treatment of DVT and PE in noncancer patients,5,11,12 evidence on their efficacy and safety in cancer patients was lacking. Recently, the results of 2 randomized trials comparing DOACs to LMWH for the treatment of cancer-associated thrombosis were published. The Hokusai VTE Cancer trial randomized 1050 patients to oral edoxaban, a direct oral factor Xa inhibitor, or subcutaneous dalteparin, a LMWH, for the treatment of cancer-associated thrombosis.13 The trial used an open-label, blinded end point, noninferiority design and included patients with both incidental and symptomatic VTE. Edoxaban was given at a once-daily 60-mg dose after at least 5 days of LMWH therapy. Dalteparin was given at an initial 200 IU/kg once-daily dose followed by a 150 IU/kg once-daily dose after the first month. Treatment duration was for a minimum of 6 and up to 12 months. Edoxaban was shown to be noninferior to dalteparin for the composite primary outcome of first recurrent VTE or major bleeding episode. The outcome occurred in 67 of 522 patients (12.8%) with edoxaban and in 71 of 524 patients (13.5%) with dalteparin in the 12 months following randomization (hazard ratio [HR], 0.97; 95% CI, 0.7-1.36; P = .006 for noninferiority). Compared with dalteparin, the absolute risk of recurrent VTE was 3.4% lower with edoxaban (HR, 0.71; 95% CI, 0.48-1.06; P = .09), whereas the risk of major bleeding was 2.9% higher (HR, 1.77; 95% CI, 1.03-3.04; P = .04). The reported discrepancy in major bleeding episodes was mainly due to upper gastrointestinal bleeding in patients with gastrointestinal cancer. A post hoc analysis showed that, in these patients, the risk of major bleeding was 12.5% with edoxaban and 3.6% with dalteparin (HR, 4.0; 95% CI, 1.5-10.6; P = .005).14 The bleeding complications occurred in all types of gastrointestinal cancers (esophageal, gastric, colorectal, hepatobiliary, and pancreas) and in patients with both resected and unresected tumors.
SELECT-D was a randomized, open-label, pilot trial including 406 cancer patients with acute VTE randomized to oral rivaroxaban, a factor Xa inhibitor, or dalteparin for a treatment duration of 6 months.15 Rivaroxaban was given at a dose of 15-mg twice daily for the initial 3 weeks followed by 20-mg once-daily dosing thereafter. At 6 months, the cumulative incidence of recurrent VTE was 4% with rivaroxaban and 11% with dalteparin (HR, 0.43; 95% CI, 0.19-0.99). Adjudication was performed by a central committee unaware of the treatment allocation after study completion, although adjudication of recurrent VTE events was not prespecified in the study protocol. Major bleeding cumulative incidence was 6% in the rivaroxaban group and 4% in the dalteparin group (HR, 1.83; 95% CI, 0.68-4.96). Patients with esophageal or gastroesophageal cancer experienced more major bleeding events with rivaroxaban compared with dalteparin (36% vs 11%, respectively). Similarly, a majority of the clinically relevant nonmajor bleeding (CRNMB) events in patients treated with rivaroxaban involved the gastrointestinal tract or urinary system. The data safety monitoring committee of the SELECT-D trial noted a nonsignificant increase in major bleeding events, and patients with these cancers were subsequently excluded from enrollment toward the end of the study.
A systematic review and meta-analysis combining the results of the Hokusai VTE Cancer and SELECT-D trials reported a lower rate of recurrent VTE among patients with cancer-associated thrombosis using DOACs as compared with those using LMWH (RR, 0.65; 95% CI, 0.42-1.01).16 However, the 6-month major bleeding rate was higher (RR, 1.74; 95% CI, 1.05-2.88) in patients on DOACs.16
Considerations
Taken together, the DOACs, edoxaban and rivaroxaban, seem to be an acceptable alternative to LMWH for the treatment of VTE in cancer patients. However, several factors need to be considered when tailoring anticoagulation management in a patient with cancer-associated thrombosis.
Patients’ preference needs to be the foremost important factor to include in the decision process. Qualitative research including patients with cancer-associated VTE suggested that the most important attribute from the patient’s perspective is related to potential delays or drug-drug interactions with cancer-related therapies. Other important factors include the efficacy and safety of the anticoagulation management followed by the route of administration.17 Most patients find tablets more convenient, but LMWH remains acceptable in the context of cancer and its treatment.18 All of these attributes should be discussed with the patient to reach a well-balanced shared decision.
Drug-drug interactions are important factors to consider as systemic cancer-related therapies may interfere with DOACs.19 Potent inhibitors or inducers of P-glycoprotein and cytochrome p450 CYP3A4 are known to influence the metabolization of DOACs and thereby potentially alter their efficacy and/or safety profiles (Table 2).19,20 As the extent to which these agents influence DOAC plasma concentrations is unknown, caution is warranted and LMWH might be a preferred anticoagulant agent in case of concomitant treatment with 1 of these agents. In case of treatment with edoxaban and concomitant potent P-glycoprotein inhibitor, a reduced dose of edoxaban is indicated (30 mg once daily).
Cancer-therapy–specific inhibitors and inducers of CYP3A4 and P-glycoprotein
| Cancer-related therapies . | Cytochrome p450 CYP3A4 . | P-glycoprotein . |
|---|---|---|
| Anthracyclines | ||
| Doxorubicin | ↓ | ↑ |
| Idarubicin | ↓ | |
| Antimycotic agents | ||
| Vinblastine | ↓ | ↑ |
| Vincristine | ↓ | |
| Vinorelbine | ↓ | |
| Paclitaxel | ↑ | |
| Topoisomerase inhibitors | ||
| Topotecan | ↓ | |
| Etoposide | ↓ | ↑ |
| Alkylating agents | ||
| Cyclophosphamide | ↓ | |
| Ifosfamide | ↓ | |
| Lomustine | ↓ | |
| Tyrosine kinase inhibitors | ||
| Afatinib | ↓ | |
| Alectinib | ↓ | |
| Ceritinib | ↓ | |
| Crizotinib | ↓ | ↓ |
| Dasatinib | ↓ | |
| Ibrutinib | ↓ | |
| Idelalisib | ↓ | ↓ |
| Imatinib | ↓ | ↓ |
| Lapatinib | ↓ | ↓ |
| Nilotinib | ↓ | ↓ |
| Osimertinib | ↓ | |
| Vemurafenib | ↑ | ↓ |
| Lenvatinib | ↑ | ↑ |
| Sunitinib | ↓ | |
| Vandetanib | ↓ | |
| Immune-modulating agents | ||
| Cyclosporine | ↓ | ↓ |
| Sirolimus | ↓ | |
| Temsirolimus | ↓ | |
| Tacrolimus | ↓ | ↓ |
| Methylprednisolone | ↑ | |
| Dexamethasone | ↑ | ↑ |
| Hormonal agents | ||
| Tamoxifen | ↓ | ↓ |
| Anastrozole | ↓ | |
| Bicalutamide | ↓ | |
| Enzalutamide | ↑ | ↓ |
| Abiraterone | ↓ | ↓ |
| Mitotane | ↑ | |
| Supportive care | ||
| Aprepitant | ↑↓ | |
| Fosaprepitant | ↑↓ | |
| Fentanyl | ↓ | |
| Methadone | ↓ | |
| Acetaminophen | ↓ | |
| Other | ||
| Bortezomib | ↓ | |
| Bexarotene | ↑ | |
| Venetoclax | ↓ |
| Cancer-related therapies . | Cytochrome p450 CYP3A4 . | P-glycoprotein . |
|---|---|---|
| Anthracyclines | ||
| Doxorubicin | ↓ | ↑ |
| Idarubicin | ↓ | |
| Antimycotic agents | ||
| Vinblastine | ↓ | ↑ |
| Vincristine | ↓ | |
| Vinorelbine | ↓ | |
| Paclitaxel | ↑ | |
| Topoisomerase inhibitors | ||
| Topotecan | ↓ | |
| Etoposide | ↓ | ↑ |
| Alkylating agents | ||
| Cyclophosphamide | ↓ | |
| Ifosfamide | ↓ | |
| Lomustine | ↓ | |
| Tyrosine kinase inhibitors | ||
| Afatinib | ↓ | |
| Alectinib | ↓ | |
| Ceritinib | ↓ | |
| Crizotinib | ↓ | ↓ |
| Dasatinib | ↓ | |
| Ibrutinib | ↓ | |
| Idelalisib | ↓ | ↓ |
| Imatinib | ↓ | ↓ |
| Lapatinib | ↓ | ↓ |
| Nilotinib | ↓ | ↓ |
| Osimertinib | ↓ | |
| Vemurafenib | ↑ | ↓ |
| Lenvatinib | ↑ | ↑ |
| Sunitinib | ↓ | |
| Vandetanib | ↓ | |
| Immune-modulating agents | ||
| Cyclosporine | ↓ | ↓ |
| Sirolimus | ↓ | |
| Temsirolimus | ↓ | |
| Tacrolimus | ↓ | ↓ |
| Methylprednisolone | ↑ | |
| Dexamethasone | ↑ | ↑ |
| Hormonal agents | ||
| Tamoxifen | ↓ | ↓ |
| Anastrozole | ↓ | |
| Bicalutamide | ↓ | |
| Enzalutamide | ↑ | ↓ |
| Abiraterone | ↓ | ↓ |
| Mitotane | ↑ | |
| Supportive care | ||
| Aprepitant | ↑↓ | |
| Fosaprepitant | ↑↓ | |
| Fentanyl | ↓ | |
| Methadone | ↓ | |
| Acetaminophen | ↓ | |
| Other | ||
| Bortezomib | ↓ | |
| Bexarotene | ↑ | |
| Venetoclax | ↓ |
Adapted from Short and Connors.19 Cancer-treatment specific inducers (↑) and inhibitors (↓) of cytochrome p450 CYP3A4 and P-glycoprotein are shown. DOACs are substrates to CYP3A4 and P-glycoprotein enzymes. Inducers of these enzymes may potentially increase metabolization of DOACs thereby leading to lower plasma concentrations, and inhibitors may decrease metabolization leading to higher plasma concentrations. Edoxaban, rivaroxaban, and apixaban are reported to have major interactions with the P-glycoprotein pathway. Rivaroxaban and apixaban are reported to have major interactions with the CYP3A4 pathway whereas edoxaban has been reported to have minor interactions. Dabigatran has moderate interactions with the P-glycoprotein pathway. The extent to which plasma concentrations of DOACs are influenced by inducers or inhibitors of CYP3A4 and P-glycoprotein is unknown.
The rates of major bleeding and CRNMB events seem to be higher in patients with cancer-associated thrombosis using DOACs. Therefore, bleeding risk assessment is crucial. Unfortunately, no tool is currently available to predict the risk of bleeding episodes in this specific patient population. However, patients with gastrointestinal cancer were reported to have a higher risk of bleeding complications. The underlying mechanism remains unclear, but several hypotheses have been proposed. It is possible that the presence of high gut concentrations of DOACs leads to a higher risk of bleeding complications due to local inflammation/mucositis from chemotherapy or through a direct effect on the tumor site or surgical site after tumor resection. Until we can stratify gastrointestinal cancer patients according to their underlying risk of bleeding complications, the use of DOACs should be carefully considered in this patient population by balancing patients’ preference and the risk of bleeding, considering at least age, previous bleeding episodes, anemia, thrombocytopenia, and renal function. The use of DOACs in patients at high risk of urothelial bleeding complications should also be carefully evaluated. The SELECT-D pilot trial reported more urothelial CRNMB episodes in patients on rivaroxaban. Furthermore, previously reported observational studies assessing the role of DOACs for the management of cancer-associated thrombosis have excluded patients with urothelial tumors or those with nephrostomy tubes.21
The recent guidance statement of the Scientific and Standardization Committee on Haemostasis and Malignancy of the International Society on Thrombosis and Haemostasis recommends shared decision-making with the patient and suggests the use of specific DOACs (edoxaban or rivaroxaban) for cancer patients with an acute diagnosis of VTE, a low bleeding risk, and no drug-drug interactions; LMWH is suggested for those with a high risk of bleeding, including those with thrombocytopenia.22,23 Although VKAs are still extensively prescribed for cancer-associated VTE,24 their use should be discouraged for the acute treatment of cancer-associated thrombosis, particularly in the first 3 months. Use of a VKA should be reserved for patients for whom LMWHs/DOACs are contraindicated, unaffordable, or unavailable, or in those patients currently treated and stable on this agent.
Back to case 1
Acute treatment
The patient is not currently receiving any cancer-specific therapies. He denied previous bleeding episodes and indicated that he would prefer oral over parenteral therapy. Body weight was 78 kg and renal function was within normal limit with a creatinine clearance of 80 mL/min. The patient was treated with 5 days of therapeutic LMWH (enoxaparin 1 mg/kg subcutaneously; twice-daily dose) followed by edoxaban 60 mg once daily for a planned minimal duration of 6 months.
Patient follow-up
After 6 months of treatment, the patient returned for outpatient follow-up. He remained on anticoagulant treatment and denied any recurrent VTE or bleeding episodes. Recent laboratory investigations demonstrated elevation of the prostate-specific antigen levels, and multiple distant vertebral and pelvic bone metastases were confirmed on computed tomography (CT). Androgen-deprivation therapy in combination with docetaxel was initiated. Is extended anticoagulant therapy indicated for this patient with advanced-stage cancer?
Evidence
Most clinical practice guidelines recommend a minimum of 3 to 6 months of anticoagulant therapy and suggest extending treatment duration in patients with active cancer as they are considered at high risk of recurrent VTE.5-8 These recommendations are mostly based on expert opinion as high-quality controlled studies mostly evaluated anticoagulant therapy for a duration of 6 months. Data from 3 cohort studies suggest that extended therapy might be beneficial in selected patients. The prospective DALTECAN study evaluated the efficacy and safety of extended dalteparin therapy up to 12 months in patients with cancer-associated thrombosis. The study showed that the incidences of recurrent VTE and major bleeding episodes were similar during the extended treatment period (ie, beyond 6 months), suggesting an ongoing efficacy and safety profile of anticoagulation treatment beyond the initial 6-month period.25 Similarly, the TiCAT study, which evaluated the safety of tinzaparin in 247 patients with cancer-associated thrombosis, reported no significant difference in recurrent VTE or clinically relevant bleeding between months 1 to 6 and months 7 to 12.26 A retrospective cohort study reported that the risk of recurrent VTE following discontinuation of anticoagulation was higher in cancer patients with active cancer compared with those with cured cancer (19 per 100 patient-years vs 3.2 per 100 patient-years, respectively), suggesting that extended treatment may be warranted in active cancer patients.27 Finally, the Hokusai VTE Cancer trial demonstrated acceptable efficacy and safety profiles over the 12-month treatment period. However, rates of recurrent VTE and major bleeding beyond the initial 6 months are not currently reported.13
Considerations
The decision to stop or continue anticoagulation therapy after an initial treatment period of 3 to 6 months should be based on the balance between the risk of recurrent VTE and bleeding complications in combination with patient’s preference, life expectancy, and treatment costs. Expert consensus suggests that anticoagulant treatment should be continued in patients with active cancer as it is a persistent major risk factor for VTE recurrence. In the absence of a validated standardized method, VTE recurrence risk assessment should at least include patient-related factors such as immobilization or hospitalization, tumor-related factors such as tumor type and localized tumor compression, and cancer-related therapies, including chemotherapy, hormonal therapy, and the presence of an indwelling central venous catheter (CVC) (Table 1).28 Although data on extended therapy with DOACs are lacking, it is reasonable to assume that there is no need to change the choice of anticoagulant after the initial 3 to 6 months of anticoagulation therapy.5
Back to case 1
We recommend continuing anticoagulant treatment of our patient with advanced-stage prostate cancer. As there are no significant drug-drug interactions with androgen-deprivation therapy or docetaxel, and bleeding risk is low, edoxaban is continued at a 60-mg once-daily dose and the patient is given a 3-month follow-up appointment to reevaluate anticoagulation therapy.
Case 2: incidental PE
A 72-year-old woman is diagnosed with stage IIIA distal esophageal carcinoma and started on neoadjuvant radiation therapy and chemotherapy with carboplatin and paclitaxel. Two months later, a multidetector CT scan with IV contrast was performed for evaluation of treatment response. An incidental filling defect was detected in a segmental pulmonary artery of the right lower lobe. The patient reported no dyspnea, chest pain, or hemoptysis. She had an active lifestyle and denied exertional dyspnea. Recently, she experienced 2 episodes of hematemesis. Blood pressure was 142/98 mm Hg; pulse rate, 59 per minute; temperature, 36.8°C; oxygen saturation, 98%; and respiratory rate, 17 per minute. Should this patient with incidentally detected PE receive anticoagulant treatment?
Evidence
Up to 50% of all PEs in cancer patients are incidentally detected, and the prevalence of incidental PE diagnosis has been reported to be between 1% and 15% in this patient population.3 Incidental PEs are most often diagnosed on multidetector CT scans performed to assess cancer treatment response, disease staging, or routine follow-up imaging. Although PE diagnosis is unsuspected in these patients, approximately one-half of the patients report symptoms suggestive of PE or DVT.29,30 Given that the signs and symptoms of PE are not specific, they are often attributed to the cancer itself or its underlying treatment by both the clinician and the patient, not immediately leading to suspicion of PE.
Clinical practice guidelines suggest the same anticoagulant management for patients with incidentally detected PE as for patients with symptomatic PE.31,32 However, these recommendations are largely based on retrospective studies or are extrapolated from trials with symptomatic VTE patients. Several studies suggest that the clinical outcomes of cancer patients with incidental PE are like those with a symptomatic event. A retrospective cohort study that included cancer patients with incidental (n = 51) and symptomatic PE (n = 144) reported a cumulative 1-year incidence of recurrent VTE of 13.3% and 16.9%, respectively (P = .77). Similarly, the mortality was 52.9% among patients with incidental PE and 53.3% in patients with symptomatic events (P = .70).33 Although some studies reported similar findings,30,34 others suggested a better prognosis in patients with incidental compared with symptomatic PE.35,36 However, most of these studies had small sample sizes and a retrospective study design, which may partly explain the conflicting results. The Hokusai VTE Cancer trial and the SELECT-D study enrolled ∼30% and 50% of patients with incidental PE, respectively. Of the 340 patients included in the Hokusai VTE Cancer trial with incidental events, the rates of recurrent VTE and major bleeding complications were similar compared with those with symptomatic events.37
As technology evolves, multidetector CT scanners can detect smaller and smaller filling defects with higher sensitivities for subsegmental pulmonary arteries. However, the clinical relevance of PE isolated to the subsegmental arteries remains a matter of debate.38,39 The latest version of the American College of Chest Physicians clinical practice guidelines suggested that certain patients with low-risk subsegmental PE without DVT may be left untreated.5 In the setting of cancer, studies that compared the prognosis of patients with isolated subsegmental PE to those with more proximal (segmental, lobar, and central) events reported conflicting results with regard to the underlying risk of recurrent VTE and overall mortality.29,40 In clinical practice, the vast majority of clinicians would anticoagulate cancer patients with isolated symptomatic subsegmental PE as reported by 2 surveys.41,42 However, in patients with an isolated incidental single subsegmental PE, the decision to start anticoagulant treatment is more nuanced as it could unnecessarily expose patients to a risk of bleeding. Therefore, the Scientific and Standardization Committee on Haemostasis and Malignancy of the International Society on Thrombosis and Haemostasis recommends a different approach in such patients.43 As a first step, the imaging results should be reviewed with an experienced thoracic radiologist as the interobserver agreement for emboli in the most distal pulmonary arteries (subsegmental and segmental) has been reported to be poor.44,45 The second step in the diagnostic workup of a patient with incidental single subsegmental PE is bilateral compression ultrasonography of the lower extremities to detect possible incidental DVT. In those with concomitant proximal DVT, the patient should receive standard-of-care anticoagulant therapy. In patients with no DVT, it is suggested that the decision to prescribe anticoagulation should be individualized and should consider the risk of recurrent VTE and bleeding, the performance status of the patient, and patient preference. If anticoagulation is withheld, clinical monitoring of the patient is advised, including serial ultrasonography in those with distal DVT to assess thrombus extension.
Considerations
It is widely accepted that patients with an incidental PE should receive anticoagulant treatment as for symptomatic PE. In patients with isolated, incidental, single subsegmental PE without DVT on ultrasonography, a case-by-case decision about anticoagulation therapy is warranted.
Back to case 2
The patient was diagnosed with a segmental incidental PE in the context of esophageal carcinoma. Bleeding risk was considered high in this patient with gastrointestinal cancer with 2 recent bleeding episodes. After discussion, the patient was started on therapeutic doses of LMWH (dalteparin 200 IU/kg once-daily dose followed by a 150 IU/kg once-daily dose after the first month) and a close follow-up appointment was scheduled to ensure no bleeding complications.
Case 3: catheter-related thrombosis
A 58-year-old man with stage IIB non–small cell lung cancer presents with a 4-day history of pain, swelling, and redness of the left upper extremity. A peripherally inserted central catheter (PICC) line was inserted 2 weeks ago for administration of chemotherapy. One week after initiating chemotherapy, the patient started having progressive symptoms of the left arm. He denies worsening of dyspnea or chest pain. On examination, there is redness and edema of the entire left arm. There are no signs of infection. Ultrasonography reports obstructive nonfilling defects in the axillary and brachial veins, and Doppler reports no flow within the subclavian vein. How should we treat this patient with catheter-related proximal upper extremity DVT?
Evidence
CVCs (implanted port, centrally inserted catheter, or PICC) are often used for long-term chemotherapy or parenteral nutrition in cancer patients and may be complicated by catheter-related thrombosis, mostly in the upper extremities. The reported rates of catheter-related DVT in the literature vary widely due to differences in study design, population, catheter type, diagnostic tests, and follow-up duration. In patients carrying a CVC, the risk of symptomatic catheter-related DVT has been reported to be between 1% and 5%, whereas the rate of asymptomatic events can be as high as 50%.46,47 Risk factors for catheter-related DVT may include intrinsic factors such as CVC size and type, tip location, side of placement, and extrinsic factors including inherited thrombophilia, previous VTE, and metastasized cancer.47,48 However, most of the studies that assessed these risk factors have significant limitations, including small sample size, retrospective design, and heterogeneity with regard to outcomes and procedures.
The American College of Chest Physicians guideline published in 2012 suggests that the catheter should remain in situ as long as it is functional and there is an ongoing need for the catheter, which is also supported by recent guidance statements.8,49 Anticoagulant therapy is recommended for 3 to 6 months, regardless of whether the catheter is removed, and continued treatment is recommended for as long as the catheter remains.8,32,49
To date, no randomized trials have assessed the therapeutic management of catheter-related thrombosis. Therefore, guideline recommendations are mostly based on limited observational data or are extrapolated from evidence on the treatment of cancer-associated lower extremity DVT. A recent systematic review showed that clinical practice in the management of catheter-related thrombosis varies widely with regard to anticoagulant agents and treatment duration.50 A prospective pilot study suggested that VKA is an effective and safe management strategy for catheter-related thrombosis in cancer patients.51 Seventy-four patients were managed with dalteparin for at least 5 days followed by VKA for a total of 3 months without catheter removal. All catheters remained functional and no progressive or recurrent VTE episodes occurred, although a major bleeding occurred in 3 patients (5%). Similarly, a small retrospective cohort study of 89 patients with symptomatic catheter-related upper-extremity VTE treated with 1 month of full therapeutic dose LMWH, followed by intermediate-dose LMWH, reported no recurrent VTE and 2 major bleeding episodes at 3 months.52 Extrapolating from the data of the trials reporting the efficacy and safety of LMWHs for the management of lower extremity DVT and PE among cancer patients, most guidance documents and expert consensus will suggest LMWHs over VKAs for the treatment of catheter-related upper extremity DVT in cancer patients.8,32,49 Evidence on the efficacy and safety of DOACs in this patient population is emerging. One prospective pilot study evaluated the use of rivaroxaban in 70 cancer patients with catheter-related upper extremity DVT.53 Rivaroxaban was given at an initial dose of 15 mg twice daily for 3 weeks followed by a 20-mg once-daily dose thereafter for a total duration of 3 months. Overall, all catheters remained functional, 1 patient had recurrent VTE (fatal PE) (incidence rate, 1.43%; 95% CI, 0.25-7.66), and 11 bleeding events occurred in 9 patients (incidence rate, 12.85%; 95% CI, 6.9-22.7). Therefore, the safety of DOACs for the management of catheter-related upper-extremity DVT in cancer patients remains unclear.
Considerations
Keeping the catheter in place if it is functional and there is an ongoing need for the catheter is a common recommendation. However, if the catheter is not functional or improperly positioned, or in most cases of infection, removal of the catheter is recommended, and a short duration of anticoagulation (3-5 days) is suggested prior to removal (if possible).32,49,54 Anticoagulant therapy is recommended for a minimum of 3 months and should be continued for as long as the catheter remains. As the bleeding risk with DOACs for the treatment of catheter-associated thrombosis needs further assessment, LMWH may be the preferred anticoagulant option. However, DOACs may be considered a reasonable alternative after discussion with patients.
Back to case 3
The catheter remained in place as it was functional, and the patient needed ongoing chemotherapy regimens. The patient was started on therapeutic LMWH (enoxaparin 1.5 mg/kg once-daily dose) for at least 3 months. A follow-up appointment at 3 months was scheduled to evaluate whether continued anticoagulation treatment was indicated.
Conclusions and future perspectives
VTE is a common complication that is associated with significant morbidity and mortality in cancer patients. Different manifestations require specific treatment approaches as outlined in this review. Although LMWHs have been the recommended treatment of years, recent trials showed that DOACs can also be used for the management of cancer-associated thrombosis. Edoxaban and rivaroxaban have been studied in the setting of cancer-associated thrombosis, and a randomized trial evaluating apixaban is ongoing (clinicaltrials.gov NCT03045406).
Future studies should aim at stratifying patients at low and high risk of major bleeding complications with DOACs, especially in the subgroup of patients with gastrointestinal cancer, to provide clinicians with guidance on how to better tailor individual treatment regimens. Furthermore, the efficacy and safety of extended anticoagulant treatment beyond 6 months remains to be elucidated. A randomized trial is currently assessing 2 doses of apixaban for the extended treatment of cancer-associated VTE (clinicaltrials.gov NCT03080883). Moreover, although evidence on the clinical outcomes of cancer patients with incidental VTE is emerging, future studies need to include assessment of the safety of withholding anticoagulant therapy in those with single subsegmental PE without proximal DVT, as the risks of anticoagulant treatment may outweigh the benefits in these patients. Finally, there are very little data to guide treatment decisions on catheter-related thrombosis in cancer patients. Management studies randomizing cancer patients to LMWHs or DOACs for this indication are needed to compare the safety of both regimens. An ongoing single-arm study is evaluating the efficacy and safety of apixaban for this indication (clinicaltrials.gov NCT03100071).
Authorship
Contribution: N.K. and M.C. both contributed to study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript.
Conflict-of-interest disclosure: M.C. reports research grants from Pfizer, Bristol-Myers Squibb, and Leo Pharma, and consultancy honoraria from Pfizer, Bayer, Sanofi, Servier, and Leo Pharma. N.K. declares no competing financial interests.
Correspondence: Marc Carrier, Department of Medicine, The Ottawa Hospital, 501 Smyth Rd, Ottawa, ON, Canada; e-mail: mcarrier@toh.ca.