In this issue of Blood, Li et al1 show that erythropoietin receptor (Epor) expression marks the central macrophage of the erythroblastic island (EBI). Treatment with Epo increases the number of EBIs as well as the number of erythroblasts surrounding the central macrophage.
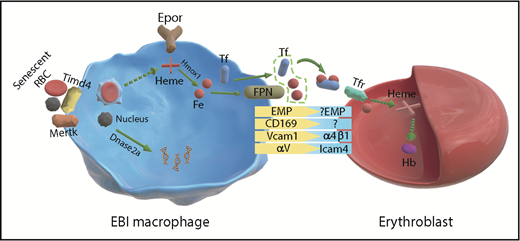
Schematic from Li et al of Epo receptor signaling in EBI macrophages and erythroblasts. Fe, iron; Hb, hemoglobin; RBCs, red blood cells.
Schematic from Li et al of Epo receptor signaling in EBI macrophages and erythroblasts. Fe, iron; Hb, hemoglobin; RBCs, red blood cells.
More than 60 years ago, Bessis first described the EBI as a specialized niche for erythroid development.2 Its basic structure of a central macrophage surrounded by developing erythroblasts reflected its role in regulating erythroid development. The central macrophage through contacts with erythroblasts provides nutrients and signals that promote erythroid differentiation. Eliminating macrophages by poisoning them with clodronate liposomes or deleting CD169+ macrophages impairs erythropoiesis.3,4 Stress erythropoiesis is particularly sensitive to these treatments because new monocytes are recruited to generate EBI macrophages.5 Failure to develop these macrophages prevents recovery from anemia.
Although the basic structure of EBIs has remained constant, the identity of the central macrophage has been constantly shifting depending on whether the EBIs were isolated from human or mouse and whether they were isolated from bone marrow, spleen, or fetal liver. Pioneering work by many laboratories characterized the adhesion molecules that mediate the interactions between erythroblasts and the central macrophage, demonstrating roles for Vcam1, Maea (Emp), and αv on the macrophage side, which bind to α4β1, Maea, and Icam4, respectively, on the erythroblast side.6 However, macrophages are not a singular population but vary greatly depending on disease state, their tissue localization, and developmental origin.7 In this background of inherent heterogeneity, Li et al identified a common marker present in EBI macrophages. They show that Epor is a marker for EBI macrophages (see figure). Using imaging flow cytometry, they demonstrate that >90% of EBI macrophages are Epor+. This expression was seen in EBI macrophages in murine bone marrow, spleen, and fetal liver EBIs as well as human bone marrow and fetal liver, suggesting that Epor+ macrophages define the EBIs. The RNA-seq analysis done on Epor+ macrophages supported this assertion as many of the genes expected to be expressed in EBI macrophages were expressed in the Epor+ population. They observed high levels of Klf1, which is known to regulate EBI macrophages as well as genes encoding the machinery for iron utilization and for engulfing nuclei extruded from developing reticulocytes.8,9
Although Epor marked the EBI macrophages, when they addressed the question of heterogeneity by examining other markers previously associated with EBI macrophages, they found that these markers were expressed in subpopulations of Epor+ EBI macrophages. This observation suggests that EBI macrophages are heterogenous, which leads to us to the question why they are heterogenous. Li et al’s analysis provided a snapshot of the EBI macrophage populations. How these populations of Epor+ macrophages correlate with the stages of erythroid development is not known. It is easy to speculate that Epo may coordinately regulate both erythropoiesis and the development and function of the EBI niche. Their data show that Epo treatment increases the number of erythroblasts associated with EBI macrophages. Future work will be needed to understand how Epo and other signals like glucocorticoids alter the niche and promote erythropoiesis.
These findings also impact the role of Epor signaling in hematologic disease. In myeloproliferative disease, Jak2 mutations will affect Epor signaling in macrophages as well as the progenitors. Recent work showing expression of Jak2V617F in myeloid cells leads to polycythemia and myeloproliferative disease.10 The role of macrophage Epor signaling in disease progression and pathology is not known and represents an additional target for therapy. Furthermore, the use of rhEpo in the treatment of anemia will impact both the niche and the progenitor cells. Failure to respond to this therapy may reflect defects in macrophages. Use of additional agents to improve macrophage responses could be key aspects to improving the response of anemic patients.
In summary, Li et al have defined the EBI macrophage as an Epor+ macrophage. These data set the stage for new avenues of research into the development and function of the EBI niche at steady state and in response to anemic stress and show us that signaling by niche macrophages can affect disease progression and pathology, which must be taken into account when developing new treatments for anemia.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal