Platelet count (PC) and mean platelet volume (MPV) are critical measurements when considering the role of platelets in hemostasis and thrombosis, abnormalities in PC and MPV have been shown to be predictors of ischemic cardiovascular disease. Ischemic vascular disease has a known genetic component, and we and others have shown high heritability for both PC and MPV. Many inherited, and most immune, thrombocytopenias show an inverse relationship between PC and MPV, and such a relationship has also been reported in healthy subjects. Although genome-wide association studies have identified numerous single nucleotide variants in protein-coding genes associated with PC and MPV (Geiger Nat Genet 2011), non-coding genes have had rather little consideration. The goal of our studies was to identify microRNAs (miRs) regulating PC and MPV and to begin to understand the molecular mechanisms of this inverse platelet number-volume relationship.
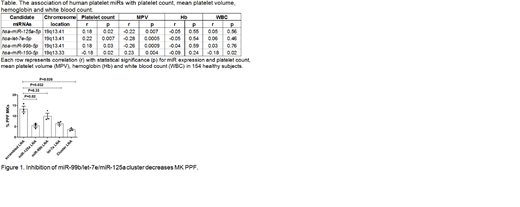
Initially, we queried our Platelet RNA and Expression-1 data set and observed that PC and MPV are inversely correlated in these 154 healthy subjects (r=-0.45, p=3.98E-09). We next determined which of the 734 platelet-expressed miRs were significantly (p<0.05) correlated with both PC and MPV. Of these miRs, only miR-125a-5p, miR-99b-5p, let-7e-5p and miR-150-5p had an inverse relationship with PC and MPV. The Table shows the correlations between these miRs and complete blood count data. Subsequent studies focused on miR-125a-5p, miR-99b-5p, let-7e-5p because they (1) were associated with only platelet parameters and not WBC or Hb, (2) were more highly expressed than miR-150-5p and (3) showed the same directionality (e.g., positive r value for PC). We were also intrigued by the fact that these 3 miRs exist as a genomic cluster within 727 bp on chr19q13.41. The expression of each of the 3 miRs in this cluster was strongly correlated with each other in platelets (r=0.93, p=1.02E-68 for miR-125a and let-7e; r=0.91, p=8.53E-61 for miR-99b and let-7e; r=0.89, p=1.19E-52 for miR-125a and miR-99b), consistent with a single, polycistronic transcript containing all genes.
To begin to address the potential functionality of this miR cluster, we overexpressed and knocked down their expression in megakaryocytes (MKs), since MK proplatelet formation (PPF) is a necessary step in the generation of peripheral blood platelets. We utilized human umbilical cord blood derived CD34-positive hematopoietic stem and progenitor cells differentiated along the MK lineage with thrombopoietin. Quantification of miR levels with qPCR demonstrated all 3 miRs in the cluster progressively increased from culture day 6 to day 9 to day 13 (the time during which MKs mature in culture). Compared to a scrambled control, when miR activity was knocked down with locked nucleic acids (LNAs), we observed a 61% reduction in PPF for miR-125a-5p (p<0.05), a 54% reduction for let-7e-5p (p<0.05) and a ~28% reduction for miR-99b-5p (p=0.33). PPF was quantified blinded as to treatment group. When all 3 miRs in the cluster were inhibited simultaneously by LNAs, we observed a 74% reduction in PPF (p<0.05) (Figure 1). LNA inhibition of two miRs not associated with PC or MPV did not alter MK PFF. To date, we have only tested the effect of overexpression of one miR, but observed that lentiviral overexpression of miR-125a-5p induced an increase in MK PPF (p<0.05). In summary, we have identified a genomic cluster of miRs (miR-99b/let-7e/miR-125a) that regulates PPF in cultured human MKs and whose expression is positively correlated with human PC and negatively correlated with MPV. We speculate that platelet mass homeostasis, which is determined by PC and MPV, may be controlled in part by this novel genomic locus.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal