INTRODUCTION:Cancer treatment-related thrombocytopenia is a serious problem in patients treated for cancer. Literature indicates the incidence of chemotherapy and radiation induced thrombocytopenia can be as high as 50%, based on underlying cancer type and treatment. This study describes patients treated with eltrombopag, a thrombopoietin receptor agonist, for cancer treatment -related thrombocytopenia.
METHODS: Patients, who initiated treatment with eltrombopag and met all inclusion criteria, were identified from the Optum EHR database between 1st Jan 2009 to 31st Mar 2018. The earliest order date for eltrombopag was considered the index date. Inclusion criteria were: 18 years of age and older; male or female; diagnosis of solid or lymphoid malignancy prior to the index date; receipt of systemic chemotherapy 4 months prior or 1 month after the index date; platelets 100,000/mm3 pre index date; no evidence of prior use of eltrombopag; no evidence of therapy with rituximab; and, active in the database for at least 4 months prior to and at least 14 days following index date. Outcomes evaluated were (1) pre-index date minimum and mean platelet values, (2) post index date maximum and mean platelet values during the index chemotherapy cycle of eltrombopag treatment and line of therapy (LOT) time periods.
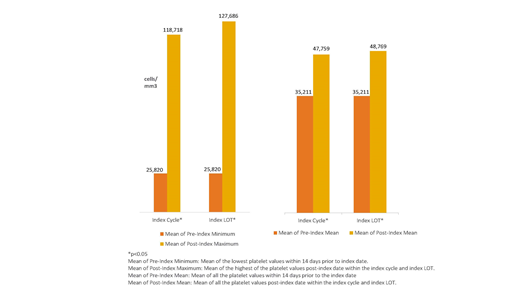
RESULTS: The study cohort consisted of 107 patients, with mean age of 63.7 years (SD: 17.9), 59.8% male, 40.2% female, and 32.7% had commercial insurance. The mean Charlson Comorbidity index (CCI) score was 4.0 (SD: 2.6). Top cancer diagnoses were malignant neoplasms of lymphoid and histiocytic tissue (14.0%), malignant neoplasm of female breast (9.4%), and malignant neoplasm of trachea, bronchus and lung (5.6%). Top chemotherapy regimens used in the cohort were: decitabine (9.4%); azacitidine (8.5%); lenalidomide (6.6%); ruxolitinib (3.8%), and carboplatin plus gemcitabine, daunorubin plus cytarabine, hydroxyurea, and radiation (2.8% for each regimen). The mean index cycle duration was 2.3 months (SD: 2.4 months) and median was 1.2 months, while the mean index LOT duration was 3.3 months (SD: 2.6 months) and median was 3 months. The pre and post mean platelet values, shown in the figure, indicated an increase in the mean platelet values post treatment in the mean and maximum values from the mean and minimum pre-index values, within both the index cycle and the LOT treatment period.
CONCLUSIONS:The results of this retrospective analysis of patients with cancer treatment-related thrombocytopenia treated with eltrombopag are consistent with eltrombopag's known profile in improving platelet counts, as evidenced for other approved indications. Further studies are needed to determine the specific patient population who will benefit most from eltrombopag in this type of secondary thrombocytopenia.
Figure1: Pre and Post Index Cycle and LOT Mean Platelet Values
Pawar:Novartis: Consultancy. Velcheti:AstraZeneca: Consultancy; Foundation Medicine: Consultancy; Merck: Consultancy; Boston Scientific: Consultancy; BMS: Consultancy; Novartis: Consultancy; Reddy Labs: Consultancy; Alkermes: Consultancy; Genentech: Consultancy. Lal:Optum: Employment. Le:Optum: Employment. Andrade:Optum: Employment. Desai:Precision Xtract: Employment. Patwardhan:Novartis Pharmaceuticals: Employment.
Eltrombopag for chemotherapy or radiation induced thrombocytopenia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal