Introduction: We have previously shown that a clinicogenetic risk model called the m7- Follicular Lymphoma (FL) International Prognostic Index (m7-FLIPI), which includes the mutation status of 7 genes (EZH2, ARID1A, MEF2B, EP300, FOXO1, CREBBP, and CARD11), the FLIPI, and the ECOG performance status improves risk stratification in patients with advanced stage FL receiving frontline immunochemotherapy (Pastore, 2015). The m7-FLIPI was trained on a group of patients treated with R-CHOP and validated on an independent cohort treated with R-CVP. The aim of this study was to test the prognostic utility of the m7-FLIPI in patients treated within the GALLIUM trial.
The GALLIUM trial enrolled 1202 patients with FL randomized to either receive Rituximab (R)- or Obinutuzumab (GA101, G)-based frontline treatment (Marcus, 2017). The chemotherapy consisted either of CHOP, CVP or Bendamustine and was allocated by the treating study center. All patients had previously untreated FL, advanced stage disease (stage III/IV or stage II with bulky disease) and ECOG performance status 0-2. All patients required treatment according to the GELF criteria.
Methods: We performed targeted DNA sequencing of recurrently mutated genes, including the m7-FLIPI genes from diagnostic FL biopsies. We excluded cases with no available biopsies, insufficient DNA quantity/quality, or if patients did not sign informed consent for molecular studies. 418 patients were available for evaluation of the m7-FLIPI. The m7-FLIPI was calculated as previously described (http://www.glsg.de/m7-flipi/). Investigator-assessed progression-free survival (PFS), the primary endpoint in the GALLIUM trial, was the primary endpoint of this analysis. We performed Kaplan-Meier estimation and Cox proportional hazards regression to test the prognostic utility of the m7-FLIPI.
The median follow up in the evaluable cohort was 4.7 years. 283 patients (68%) received Bendamustine (140 R, 143 G), 111 (27%) received CHOP (52 R, 59 G), and 24 (6%) received CVP (12 R, 12 G).
Results: 104 patients (25%) had high-risk m7-FLIPI. 90 out of 194 patients with high-risk FLIPI (46%) were reclassified as low-risk by the m7-FLIPI. High-risk m7-FLIPI was associated with shorter PFS in the overall cohort (HR 1.52, P=0.030). Likewise, the m7-FLIPI was prognostic in patients treated with R-based regimens (HR 1.67, P=0.037). However, it was not prognostic in patients treated with G-based regimens (HR 1.24, P=0.49). When analyzed by different chemotherapy regimens, the m7-FLIPI was prognostic in patients receiving CHOP/CVP-based treatment (HR 2.05, P=0.013), validating the results from our original publication. The m7-FLIPI outperformed the FLIPI (HR 1.34, P=0.31) and had a significantly higher C-index (m7-FLIPI C=0.69, FLIPI C=0.57, P=0.021). However, the m7-FLIPI was not prognostic in patients receiving Bendamustine-based treatment (HR 1.23, P=0.42).
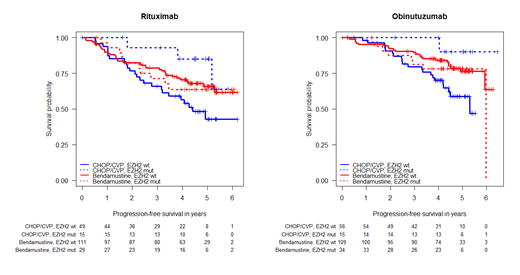
Within the m7-FLIPI, mutations in EZH2 have the highest weight of all gene mutations. In this study, we found EZH2 mutations in 93 cases (22%). EZH2 mutations were associated with longer PFS (HR 0.25, P=0.0036) in CHOP/CVP-treated patients, but not in Bendamustine-treated patients (HR 1.11, P=0.71). Interaction analysis between the chemotherapy regimen and EZH2 mutation status showed a significant interaction term of 1.49 (P=0.011) for EZH2 mutations and Bendamustine. This increased the previously favorable HR in EZH2 unmutated patients (HR 0.55 for Bendamustine vs CHOP/CVP) to an unfavorable HR of 2.42 in EZH2 mutated patients. Overall, patients with EZH2 mutant FL appeared to benefit more from CHOP/CVP, whereas patients without EZH2 mutations had longer PFS with Bendamustine-based regimens, regardless whether chemotherapy was combined with R or G (see Figure).
Conclusions: Here, we validated the prognostic utility of the m7-FLIPI for patients treated with R-CHOP/CVP. However, the m7-FLIPI was not prognostic in patients treated with Bendamustine-based regimens. While EZH2 mutation status was associated with longer PFS in patients receiving CHOP/CVP regimens (with either R or G), it did not impact treatment outcome of patients treated with Bendamustine, suggesting that EZH2 mutation status is a predictive marker for differential efficacy of the chemotherapy regimen. If confirmed, the EZH2 mutation status might be a highly useful biomarker to guide the selection of the preferred upfront chemotherapy.
Oestergaard:F. Hoffmann-La Roche: Employment. Knapp:F. Hoffmann-La Roche Ltd: Employment. Mundt:F. Hoffmann-La Roche: Employment. Fitzgibbon:Gilead: Speakers Bureau; Epizyme: Membership on an entity's Board of Directors or advisory committees, Research Funding. Klapper:Roche, Takeda, Amgen, Regeneron: Honoraria, Research Funding. Marcus:Roche / Genentech: Honoraria, Speakers Bureau; Gilead: Consultancy. Davies:Karyopharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Acerta Pharma: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; GSK: Research Funding; MorphoSys AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT Therapeutics: Honoraria, Research Funding; BioInvent: Research Funding. Herold:Roche: Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Gilead: Honoraria; Celgene: Honoraria. Hiddemann:F. Hoffmann-La Roche: Research Funding. Unterhalt:F. Hoffmann-La Roche: Research Funding. Hoster:Roche Pharma AG: Other: Travel support; Janssen: Research Funding. Weigert:Novartis: Research Funding; F. Hoffmann-La Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal