Introduction. Acquired severe aplastic anemia (SAA) is a life-threatening disorder characterized by severe progressive pancytopenia and hypocellular bone marrow. The etiology of acquired SAA is not understood but believed to be related to abnormal immune responses to environmental exposures. We conducted a genome-wide association study (GWAS) to identify common germline variants associated with SAA.
Methods. We identified 895 patients with SAA who underwent related or unrelated hematopoietic cell transplant (HCT) with clinical data and pre-HCT blood samples available in the Center for International Blood and Marrow Transplant Research (CIBMTR) database and biorepository. Pre-HCT DNA was extracted from blood of patients with SAA and genome-wide genotyping was conducted using the Illumina OmniExpress array. We excluded 93 inherited bone marrow failure cases. The SAA cases were grouped cases into discovery and validation sets based on time of batch sample receipt. Analyses were limited to patients of European ancestry based on principal component analyses to minimize the potential effect of population stratification. Controls were genomically matched and selected from previously scanned cancer-free subjects at the Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, NCI. The final analysis included 534 acquired SAA cases (359 in the discovery set and 175 in the validation set), and 2,455 controls (1,396 in the discovery set, and 1,059 in the validation set).
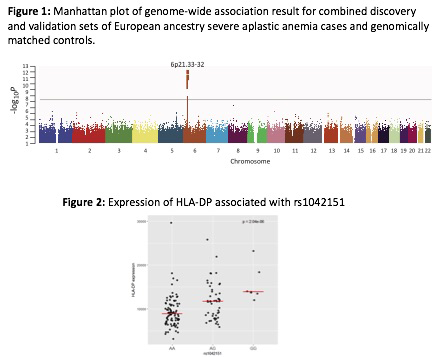
Results. Patients with SAA in this study received HCT between 1989-2015 at a median age of 21 years, 56% were male, and the median time between SAA diagnosis and HCT was 11 months. Strong genome-wide association signals were identified across the human leukocyte antigen (HLA) genes encoded at the major histocompatibility complex (MHC) on chromosome 6p21 (Figure 1). The top SNP was located in the P4 binding pocket of the HLA class II gene HLA-DPB1(rs1042151A>G, p.Met105Val, pooled-odds ratio [OR]=1.75, 95% confidence interval [CI]=1.50-2.03, p=1.94x10-13). The expression of HLA-DP in CD19+ cells from 175 healthy donors was significantly different by rs1042151 A>G genotype (p=2.04x10-6) (Figure 2). A second SNP near HLA-B, rs28367832G>A, also reached genome-wide significance (pooled-OR=1.49, 95% CI=1.22-1.78, p=7.27x10-9). Copy-number variant analysis and next generation sequencing also identified somatic, clonal copy-neutral loss-of-heterozygosity affecting class I HLA genes in 8.6% of the SAA cases and none of the controls.
Conclusion. This SAA GWAS identified strong association signals between common germline genetic variants in HLA class I and II genes and SAA. The main SNP is associated with changes in HLA-DP expression suggesting a key role for this locus in SAA etiology. This study adds further evidence to the connection between SAA and immune dysregulation.
Cerhan:Janssen: Membership on an entity's Board of Directors or advisory committees; NanoString: Research Funding; Celgene: Research Funding. Lee:Incyte: Research Funding; Syndax: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Takeda: Research Funding; Kadmon: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; AstraZeneca: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal