
Introduction: Polatuzumab vedotin (Pola) combined with obinutuzumab (G) demonstrated activity and tolerability in a Phase Ib/II trial of patients (pts) with relapsed/refractory (R/R) follicular lymphoma (FL; Phillips et al. Blood 2016). In addition, the doublet combination of G plus lenalidomide (Len) showed favorable activity and an acceptable safety profile in a Phase II study of pts with R/R FL (Morschhauser et al. Lancet 2019). We sought to determine whether Pola-G-Len might further enhance anti-tumor response in R/R FL. Here, for the first time, we present the full primary analysis of efficacy and safety data from a Phase Ib/II study (GO29834; NCT02600897) of Pola-G-Len in pts with R/R FL.
Methods: GO29834 is an open-label, multicenter study of pts with R/R FL (excluding grade 3b) who had received ≥1 prior anti-CD20-containing chemo-immunotherapy regimen. An initial 3+3 dose-escalation phase to define the recommended Phase II dose (RP2D) combination for Pola + Len was expanded into Phase II. Pts in the expansion cohort received induction treatment with six 28-day cycles of: G 1000mg IV (Cycle [C]1: Day [D] 1, D8, D15; C2-6: D1); Pola 1.4mg/kg IV (D1), and Len 20mg PO (D1-21). Responders received maintenance treatment for 24 months (G 1000mg on D1 every 2 months and Len 10mg on D1-21 during Months 1-12). The primary endpoint was complete response (CR) at end of induction (EOI), as determined by the Independent Review Committee (IRC) based on positron emission tomography-computed tomography (PET-CT) scans (by modified Lugano 2014 criteria). In addition, progression-free survival (PFS) was determined by the investigator.
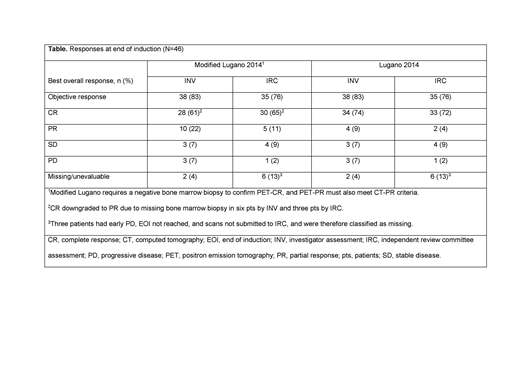
Results: At the time of the primary analysis (March 12, 2019), a total of 56 pts from the Phase Ib and Phase II populations were enrolled and had entered induction; the median duration of follow-up was 11.79 months. Baseline characteristics were: median age, 62 years; male, 59%; Ann Arbor Stage III-IV, 88%; Follicular Lymphoma International Prognostic Index high-risk (≥3), 55%; bulky disease (≥7cm), 16%; ≥2 prior lines of therapy, 77%; refractory to last line of prior regimen, 50%; and refractory to last line of anti-CD20 treatment, 45%. All pts had at least one adverse event (AE), 31 (55%) had a serious AE, and 44 (79%) had a grade 3-4 AE. The most common grade 3-4 AEs were neutropenia (28 pts, 50%), thrombocytopenia (13 pts, 23%), infections (9 pts, 16%), and anemia (8 pts, 14%). AEs leading to a dose reduction or interruption of any drug occurred in 19 (34%) and 41 (73%) of pts, respectively; the majority were modifications of Len. In addition, 14 (25%) pts had an AE that led to the discontinuation of any study drug. One grade 5 AE was reported (septic shock); however, it was not considered to be related to study treatment as the pt was receiving a new anti-lymphoma treatment after experiencing disease progression (PD). In the primary efficacy population (n=46), the IRC-assessed modified Lugano objective response rate was 76%, with a CR rate of 65% (Table). A sub-group analysis showed that 71% (15/21) of pts who were refractory to their last treatment achieved a CR. In total, five pts experienced PD, three in C1 or C2 and two at the month 12 response assessment. With a median follow-up duration of 11.27 months, median PFS was not reached.
Conclusions: Our study of the novel triplet combination, Pola-G-Len, demonstrates a safety profile consistent with the known profiles of the individual drugs. This first report of the full efficacy population showed high CR rates at EOI in a heavily pre-treated and refractory population, which compares favorably with currently available R/R FL therapies. These compelling findings support the further investigation of this triplet combination in a larger pt population. To determine the median PFS, a longer period of follow-up, through and beyond maintenance treatment, is ongoing.
Diefenbach:MEI: Research Funding; Trillium: Research Funding; Millenium/Takeda: Research Funding; Seattle Genetics: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Denovo: Research Funding; Genentech: Consultancy, Research Funding; Incyte: Research Funding; LAM Therapeutics: Research Funding. Kahl:Seattle Genetics: Consultancy; ADC Therapeutics: Consultancy, Research Funding; TG Therapeutics: Consultancy; BeiGene: Consultancy. Banerjee:Gilead: Other: Travel; Takeda: Other: Travel; Novartis: Other: Travel. McMillan:Sandoz: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau; Novartis: Honoraria; MSD: Honoraria; Pfizer: Honoraria, Research Funding; BMS: Honoraria; Gilead: Honoraria; Celgene: Honoraria, Speakers Bureau. Miall:F. Hoffmann-La Roche Ltd: Honoraria; Takeda: Honoraria, Other: Conference registration travel expenses. Briones:Roche: Honoraria, Research Funding. Cordoba:Janssen: Consultancy, Honoraria, Speakers Bureau; Servier: Consultancy, Honoraria, Speakers Bureau; Kyowa-Kirin: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Roche: Honoraria, Speakers Bureau; FUNDACION JIMENEZ DIAZ UNIVERSITY HOSPITAL: Employment; Celgene: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy. Hirata:F. Hoffmann-La Roche Ltd: Equity Ownership; Genentech, Inc.: Employment. Chang:Roche Canada: Employment. Musick:Roche/Genentech: Employment, Equity Ownership. Abrisqueta:Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Other: Travel, Accommodations, expenses, Speakers Bureau; Roche: Consultancy, Honoraria, Other: Travel, Accommodations, expenses, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, expenses, Speakers Bureau.
Polatuzumab vedotin (POLIVY, Genentech, Inc.) is a CD79b-directed antibody-drug conjugate. It was approved by the FDA in June 2019 in combination with bendamustine and rituximab for the treatment of adults with relapsed/refractory diffuse large B-cell lymphoma after at least two prior therapies.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal