Acute myeloid leukemia (AML) is an aggressive, heterogeneous malignancy. AML patients whose disease relapses on chemotherapy or are unfit for aggressive induction regimens have limited therapeutic options. Many patients benefit from the combination of venetoclax (BCL2i) and a hypomethylating agent (HMA) but this regimen is rarely curative. The addition of novel agents could provide improved benefit for relapsed/refractory patients.
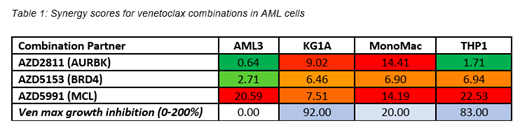
To identify such regimens, we screened a panel of 10 AML cell lines with combinations of venetoclax and novel targeted agents. The agents used spanned multiple mechanisms of action (e.g. DNA damage response, kinase signaling, pro-apoptotic agents) and are all in early clinical development. Cells were treated for 72hrs and viability was assessed by CellTiter-Glo. In several of the cell lines that were insensitive or partially sensitive to venetoclax (OCI-AML3, KG1a, MonoMac6, THP1), combinations with inhibitors of MCL1 (AZD5991), AURKB (AZD2811), and BRD4 (AZD5153) showed synergistic activity (Loewe synergy score >5, growth inhibition > 180%) (Table 1).
We next asked if these combinations were active in patient-derived xenograft (PDX) models of AML. We established an ex vivo co-culture assay using the HS-5 bone marrow stromal cell line. AML PDX cells were isolated from mouse spleens and plated in 96-well format in direct co-culture with HS-5 cells or in HS-5-derived conditioned media. Cells were treated with three doses of each monotherapy and three doses of fixed ratio combination. Replicate screens using cells from individual mice on different days confirmed data were reproducible (r2=0.687) across animals engrafted with the same PDX. Drug response was similar between conditioned media and direct co-culture assays (r2=0.81).
Venetoclax sensitivity varied across PDX models ex vivo. Notably, 2/5 PDX models screened (DFAM-68555 and DFAM-10360) were insensitive to both venetoclax and the combination of venetoclax + 5-azacytidine (HMA) ex vivo. Both models were established from untreated/1L patients and harbor TP53 mutations. Combination treatments did not add additional benefit over venetoclax monotherapy in the DFAM-10360 model. However, in DFAM-68555, AZD5153, AZD5991, and AZD2811 showed improved activity over venetoclax alone (67%, 54%, and 67% vs. 26% decrease in viability for venetoclax alone, respectively). Since combination strategies will likely be most impactful in patients refractory to or relapsed after venetoclax, we chose this venetoclax insensitive model to prioritize in vivo.
To confirm the translatability of these findings, we designed a pilot in vivo study using DFAM-68555. Mice were randomized to receive vehicle, venetoclax + HMA, or venetoclax + AZD5153 when peripheral blood disease reached ~5% (hCD45+hCD33+ cells by flow cytometry). After two weeks of dosing, animals were sacrificed to evaluate disease burden in bone marrow (sternum), spleen, and peripheral blood. The model remained insensitive to venetoclax + HMA in vivo. The combination of AZD5153 with venetoclax decreased disease burden in blood and spleen compared to vehicle (30% and 42% hCD45+CD33+ cells by flow cytometry vs 70% and 95%, respectively) with similar efficacy seen by immunohistochemistry in the bone.
Finally, we screened these venetoclax combinations in additional aggressive AML PDX models which were resistant or only partially responsive to venetoclax in vivo. Addition of AZD2811NP and AZD5991 to venetoclax was more effective than venetoclax alone and venetoclax + HMA in the bone marrow. The most active combination varied from model to model. Efficacy screening in additional models is ongoing to further build ex vivo to in vivo translation and prioritize development of specific combinations. Also ongoing is genomic and transcriptomic profiling of these PDXs to identify potential predictive biomarkers of combination activity.
In summary, we developed an ex vivo screening platform to test clinically actionable combinations for activity in clinically relevant models. Using this platform and subsequent in vivo efficacy, we identified venetoclax combinations across multiple mechanisms (pro-apoptotic, cell cycle regulation, transcriptional regulation, DNA damage response) with activity in venetoclax-insensitive models. These results suggest potential therapeutic options to explore clinically for AML patients.
Andersen:AstraZeneca: Employment. Christie:AstraZeneca: Employment. Rosen:Astrazeneca: Employment. Maratea:AstraZeneca: Employment. Hattersley:AstraZeneca: Employment. Travers:AstraZeneca: Employment. Cidado:AstraZeneca: Employment. Pulukuri:AstraZeneca: Employment. Saeh:AstraZeneca: Employment. Clark:AstraZeneca: Employment, Equity Ownership. Reimer:AstraZeneca: Employment. Mettetal:AstraZeneca: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal