As ~90% children with Acute Lymphoblastic Leukemia (ALL) are now cured, the next challenge of contemporary ALL therapy is curing with less toxicity. To reduce toxicity, the Malaysia-Singapore ALL 2003 (MS2003) study lowered the intensity of induction in Standard(SR)/Intermediate Risk(IR) patients by removing Daunorubicin. However, toxicities causing prolonged delays during Consolidation and Delayed-Intensification(DI) remained major problems. To address these delays, the MS2010 study: (1) Replaced half of all cytarabine blocks with 2 doses of Vincristine(VCR) per block (2) Implemented Doxorubicin-free DI Protocol V (3) Added 1 dose of L-Asparaginase(L-Asp) to each DI block. Here, we report the significant improvements in MS2010 compared to MS2003 in terms of treatment-related toxicities.
Methods
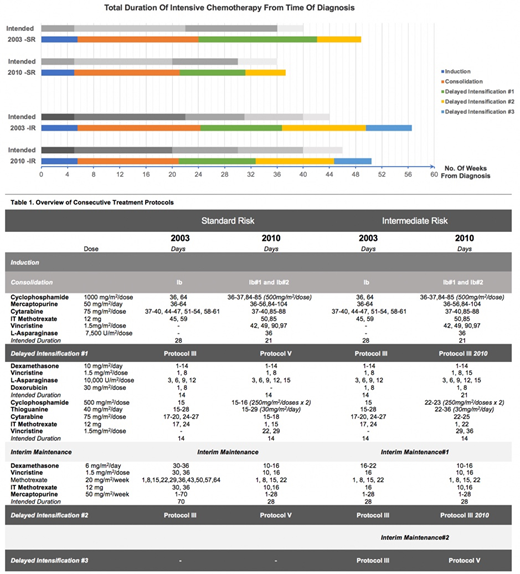
MS2003 (Yeoh AEJ et al. J Clin Oncol 2012) started with a 3 drug induction for non-HR patients in a BFM ALL-IC 2002 backbone where SR consisted of 2x Protocol III and IR consisted of 3x Protocol III. In MS2010 (Yeoh AEJ et al. J Clin Oncol 2018), SR consisted of 2x Protocol V and IR consisted of 2x Protocol III + 1x Protocol V (Table 1).
We performed a per-protocol analysis of 315 children with de novo SR/IR ALL, treated on MS2003 (n=183) and MS2010 (n=132) in 2 centers in Singapore, with a median follow up of 10.7 and 6.4years respectively. We analyzed clinical toxicity data, focusing on hospitalizations for fever (regardless of neutrophil count). Mean differences were adjusted for baseline characteristics including sex, race, age and Down syndrome.
Results
Overall decreased hospitalization stay and phase delays.
In MS2010, the total duration of hospitalization for fever are fewer and shorter; this is most apparent for DI phases. For SR group, total length of stay during DI decreased from 13.7 to 6.1 days with adjusted(adj.) δ=7.0 days (95% CI 3.5-10.5, p<0.001). For IR group, this was from 19.1 to 11.5days with adj.δ=5.2 days (95% CI 0.2-10.3, p=0.042).
In MS2010, overall delays in treatment are significantly reduced -- SR: From 65.9 to 16.4days, adj.δ=48.8 days(95% CI 39.8-57.8, p<0.001); IR: From 106.9 to 23.6days, adj.δ=80.9days (95% CI 68.4-93.5, p<0.001). The reduction in delays are mainly seen during Consolidation and DI. Overall, patients also completed intensive chemotherapy in a much shorter duration of time in MS2010 (see Table.1). Total ICU admissions were decreased (SR: 9.0% to 4.7% p=0.346 ; IR: 13.5% to 5.9% p=0.57). Total episodes of bacteremia were also decreased (SR: 31.2% to 18.75% p=0.708 ; IR 31.4% to 26.5% p=0.23).
MS2010 Protocol III had 5 doses of L-Asp compared to 4 doses of L-Asp in MS2003 Protocol III. Although L-Asp is generally considered less myelosuppressive, there was a significant increase in the number of hospitalizations during MS2010 Protocol III blocks. This translated to longer hospitalizations per patient in MS2010 during those specific phases (III#1: adj.δ=-1.6 days 95% CI -2.8 to -0.3 p=0.017; III#2: adj.δ=-1.9 days 95% CI -3.2 to -0.6 p=0.006). These increases in hospitalizations in MS2010 Protocol IIIa were most likely due to an additional single dose of L-Asp.
Equivalent survival outcomes
Based on a per-protocol analysis, 5 year Event-Free survival(EFS) outcomes between the cohorts were equivalent. In the SR cohort, 5yr EFS was 93.6% (MS2003) vs. 95.5% (MS2010) p=0.685. In the IR cohorts, 5yr EFS improved from 89.4% (MS2003) to 95.6% (MS2010) p=0.151, although statistical significance was not reached.
Overall, significant improvements: reduction in hospitalisation stays and reduction in treatment delays were observed in MS2010. This was achieved through a combination of (1) Replacing half of all cytarabine blocks with 2 doses of VCR per block; and (2) New DI Protocol V with no Anthracyclines. Despite the AIEOP-BFM 2000 experience of increased toxicity with Protocol III, our modifications to consolidation Ib and Protocol III have led to less phase delays, tolerable toxicities with comparable outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal