Acute myeloid leukemia (AML) is an aggressive and heterogeneous clonal disorder of hematopoietic stem/progenitor cells (HSPCs). Leukemogenesis is characterized by rapid growth and impaired differentiation of leukemic cells, which progressively occupy and likely alter bone marrow (BM) niche where normal HSPCs reside [Kumar B., et al. Leukemia. 2018]. The homing and adhesion of AML cells to the BM niche is critical for its pathogenesis and involves many adhesion molecules, chemokine receptors, their ligands and signaling cascades. In the healthy context, the CXC chemokine receptor type 4 (CXCR4) expressed on HSPCs and its ligand, stromal derived factor-1 alpha (SDF-1α) produced by BM niche, are key mediators of HSPC-BM niche interaction inducing the homing, quiescence and survival of HSPCs, and the regulation of the HSC pool amount [Sánchez-Aguilera A., et al. Cell Mol Life Sci. 2017]. In AML context, where CXCR4/SDF-1α axis is deregulated, growth, BM niche-homing and adhesion of leukemic cells are altered also inducing AML relapse/resistance [Sánchez-Aguilera A., et al. Cell Mol Life Sci. 2017]. Despite recent improvements in therapeutic approaches, only a fraction of AML patients is cured. Therefore, it is crucial to identify new molecular mechanisms driving the AML pathogenesis.
Extracellular vesicles (EVs) are lipidic bi-layer membrane particles released by normal and neoplastic cells. They shuttle a complex molecular cargo which reflects that of the origin cell [Caivano A., et al. Tumor Biol 2015; Caivano A., et al. Cell Oncol 2017] and which can be transferred to target cells altering their biological functions [De Luca L., et al. Oncotarget 2016; De Luca L., et al. Int J Mol Sci. 2017]. In particular, neoplasm derived EVs promote a tumor-supporting environment in non-malignant cells favoring cancer proliferation [Laurenzana I., et al. Stem Cells Int. 2018; De Luca L., et al. Expert Rev Mol Diagn. 2019].
We studied the effect of leukemia derived-EVs (LEVs) on healthy umbilical cord blood HSPCs to explore their potential role in dysregulating normal hematopoiesis. Specifically, the proliferation and differentiation, the quantitative and qualitative colony-forming features and the SDF-1α /CXCR4 mediated-migration of HSPCs induced by LEVs were investigated.
EVs were isolated from AML cell lines by centrifugation steps and analyzed for AML surface markers by flow cytometry. Next, they were co-incubated with healthy CD34+ sorted cells for 24 hours. After treatment, cell count, apoptosis, HSPC-specific cluster of differentiation marker (CD10, CD34, CD38, CD45RA, CD90, CD123) expression [Karamitros D., et al. Nat Immunol. 2018], in vitro colony-forming unit cell assay, CXCR4 levels and SDF-1α mediated-migration assay were assessed.
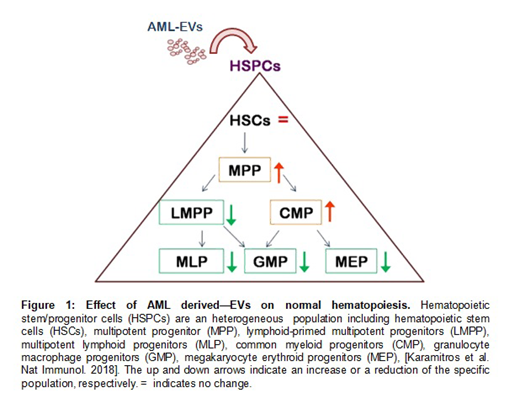
Our data showed: i) LEVs displaying specific AML surface markers; ii) a similar number of CD34+ cells in LEV treated-HSPCs, including HSCs, compared with controls; iii) no differences in apoptosis between the two groups; iv) an increase of multipotent progenitor (MPP, p=0.01) associated with a reduction of lymphoid-primed multipotent progenitors (LMPP, p=0.01) in treated cells; v) an increase of common myeloid progenitors (CMP, p=0.01), together with reduction of granulocyte macrophage progenitors (GMP, p=0.01) and megakaryocyte erythroid progenitors (MEP) in treated group; vi) a reduction of colony-forming ability, including erythroid, granulocytic and megakaryocytic colonies (p=0.03), in treated cells; vii) decreased levels of CXCR4 in LEV treated-HSPCs, that were accompanied by reduced HSPC migration mediated by SDF-1α.
Altogether, this study suggests that AML derived-EVs can dysregulate the normal hematopoiesis promoting an increase of less differentiated precursors (MPP and CMP) which is associated with a reduction of more committed myeloid progenitors (GMP and MEP) (Figure 1). Furthermore, AML derived-EVs seem to induce less attraction of HSPCs in BM niche by interfering with SDF-1α/CXCR4 axis. Strategies to block EV production and secretion, as well as EV induced-reprogramming, could be novel exciting therapeutic approaches in AML.
Musto:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal